Nylon can be used as the matrix material in composite materials, with reinforcing fibers like glass or carbon fiber; such a composite has a higher density than pure nylon. such as the silk nylon was made to replace, are also polyamides. Also, under some conditions stockings could decompose[19] turning back into nylon's original components of air, coal, and water. [20] The other was a white elastic but strong paste that would later become nylon. Its various properties also make it very useful as a material in additive manufacturing; specifically as a filament in consumer and professional grade fused deposition modeling 3D printers. If you want to know how this condensation polymerization works, click here. [33] Nylon fabric could also be itchy, and tended to cling and sometimes spark as a result of static electrical charge built up by friction. low crystallinity: PAMXD6 made from m-xylylenediamine and adipic acid; Pleats and creases can be heat-set at higher temperatures, Better weathering properties; better sunlight resistance. As of 1950, the New York Quartermaster Procurement Agency (NYQMPA), which developed and tested textiles for the army and navy, had committed to developing a wool-nylon blend.  The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. The two polymers can react with one another by transamidation to form random copolymers.[73]. When being molded, nylon must be dried to prevent hydrolysis in the molding machine barrel since water at high temperatures can also degrade the polymer. [18] DuPont's production of nylon stockings and other lingerie stopped, and most manufactured nylon was used to make parachutes and tents for World War II. Eventually, however, after experimenting with various types of metal and smoothing and polishing techniques, Augustine was also able to produce high quality nylon wound strings. organic nylon substances chemistry important hence polymeric chains hydrogen bond between By August 1945, manufactured fibers had taken a market share of 25%, at the expense of cotton. [18], Another added bonus to the campaign was that it meant reducing silk imports from Japan, an argument that won over many wary customers. [44] Nylon510, made from pentamethylene diamine and sebacic acid, was included in the Carothers patent to nylon66[23] and has superior properties, but is more expensive to make. nylon polycarbonate poly chemistry tutorial fig [40] In Britain in November 1951, the inaugural address of the 198th session of the Royal Society for the Encouragement of Arts, Manufactures and Commerce focused on the blending of textiles. You don't", "The History of Classical guitar strings", Joseph X. Labovsky Collection of Nylon Photographs and Ephemera, https://en.wikipedia.org/w/index.php?title=Nylon&oldid=1098850537, Short description is different from Wikidata, Articles containing potentially dated statements from 2020, All articles containing potentially dated statements, Articles needing additional references from February 2020, All articles needing additional references, Articles needing additional references from December 2021, Wikipedia articles needing clarification from August 2014, Creative Commons Attribution-ShareAlike License 3.0, Hexamethylene diamine (HMD): Crude oil butadiene , 1,9-diaminononane: Crude oil butadiene 7-octen-1-al 1,9-nonanedial 1,9-diaminononane, 2-methyl pentamethylene diamine: a by product of HMD production, Trimethyl Hexamethylene diamine (TMD): Crude oil propylene . Nylons are also called polyamides, because of the characteristic This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.[75].
The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. The two polymers can react with one another by transamidation to form random copolymers.[73]. When being molded, nylon must be dried to prevent hydrolysis in the molding machine barrel since water at high temperatures can also degrade the polymer. [18] DuPont's production of nylon stockings and other lingerie stopped, and most manufactured nylon was used to make parachutes and tents for World War II. Eventually, however, after experimenting with various types of metal and smoothing and polishing techniques, Augustine was also able to produce high quality nylon wound strings. organic nylon substances chemistry important hence polymeric chains hydrogen bond between By August 1945, manufactured fibers had taken a market share of 25%, at the expense of cotton. [18], Another added bonus to the campaign was that it meant reducing silk imports from Japan, an argument that won over many wary customers. [44] Nylon510, made from pentamethylene diamine and sebacic acid, was included in the Carothers patent to nylon66[23] and has superior properties, but is more expensive to make. nylon polycarbonate poly chemistry tutorial fig [40] In Britain in November 1951, the inaugural address of the 198th session of the Royal Society for the Encouragement of Arts, Manufactures and Commerce focused on the blending of textiles. You don't", "The History of Classical guitar strings", Joseph X. Labovsky Collection of Nylon Photographs and Ephemera, https://en.wikipedia.org/w/index.php?title=Nylon&oldid=1098850537, Short description is different from Wikidata, Articles containing potentially dated statements from 2020, All articles containing potentially dated statements, Articles needing additional references from February 2020, All articles needing additional references, Articles needing additional references from December 2021, Wikipedia articles needing clarification from August 2014, Creative Commons Attribution-ShareAlike License 3.0, Hexamethylene diamine (HMD): Crude oil butadiene , 1,9-diaminononane: Crude oil butadiene 7-octen-1-al 1,9-nonanedial 1,9-diaminononane, 2-methyl pentamethylene diamine: a by product of HMD production, Trimethyl Hexamethylene diamine (TMD): Crude oil propylene . Nylons are also called polyamides, because of the characteristic This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.[75]. 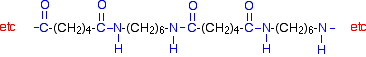 Nylon 11 and nylon 12 are the most widely used. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord. [29] Although nylon stockings already made before the war could be purchased, they were generally sold on the black market for as high as $20.[27]. Nylon 610 and 612 are the most used polymers for filaments. from the monomers adipoyl chloride and hexamethylene diamine. You'll find nylons in cars and in your home if you just look. Other nylons include copolymerized dicarboxylic acid/diamine products that are not based upon the monomers listed above. The first example of nylon, (nylon 66), was synthesized using diamines on February 28, 1935, by Wallace Hume Carothers at DuPont's research facility at the DuPont Experimental Station. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. [78] The Italian company Aquafil also has demonstrated recycling fishing nets lost in the ocean into apparel. However, polyamide is hygroscopic. It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain -pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk fibroin and the -keratins in feathers. One historian describes nylon as "an object of desire", comparing the invention to Coca-Cola in the eyes of 20th century consumers. Synthetic fibers have not dominated the market since the 1950s and 1960s. Multistranded nylon cord and rope is slippery and tends to unravel. After three years of development, Augustine demonstrated a nylon first string whose quality impressed guitarists, including Segovia, in addition to DuPont. For example, this method is applicable for preparation of nylon 1,6 from adiponitrile, formaldehyde and water. [19]:145146 The first public sale of nylon stockings occurred on October 24, 1939, in Wilmington, Delaware. Overall production of synthetic fibers, however, dropped from 63% of the worlds textile production in 1965, to 45% of the world's textile production in early 1970s. [15][42], A persistent urban legend exists that the name is derived from "New York" and "London"; however, no organisation in London was ever involved in the research and production of nylon. [56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57]. Firstly, the dimensions will change, but more importantly moisture acts as a plasticizer, lowering the glass transition temperature (Tg), and consequently the elastic modulus at temperatures below the Tg[88]. [47][48] Nylon's commercial announcement occurred on October 27, 1938, at the final session of the Herald Tribune's yearly "Forum on Current Problems", on the site of the approaching New York City world's fair. nylon making chloride diamine diacid mechanism reaction monomers hexamethylene acid adipic pslc ws Nylon has been used for meat wrappings and sausage sheaths. [78] US clothing company Patagonia has products containing recycled nylon and in the mid-2010s invested in Bureo, a company that recycles nylon from used fishing nets to use in sunglasses and skateboards. It's a lot like nylon 6,6 except that The nylon in the pictures on this page is called nylon
Nylon 11 and nylon 12 are the most widely used. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord. [29] Although nylon stockings already made before the war could be purchased, they were generally sold on the black market for as high as $20.[27]. Nylon 610 and 612 are the most used polymers for filaments. from the monomers adipoyl chloride and hexamethylene diamine. You'll find nylons in cars and in your home if you just look. Other nylons include copolymerized dicarboxylic acid/diamine products that are not based upon the monomers listed above. The first example of nylon, (nylon 66), was synthesized using diamines on February 28, 1935, by Wallace Hume Carothers at DuPont's research facility at the DuPont Experimental Station. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. [78] The Italian company Aquafil also has demonstrated recycling fishing nets lost in the ocean into apparel. However, polyamide is hygroscopic. It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain -pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk fibroin and the -keratins in feathers. One historian describes nylon as "an object of desire", comparing the invention to Coca-Cola in the eyes of 20th century consumers. Synthetic fibers have not dominated the market since the 1950s and 1960s. Multistranded nylon cord and rope is slippery and tends to unravel. After three years of development, Augustine demonstrated a nylon first string whose quality impressed guitarists, including Segovia, in addition to DuPont. For example, this method is applicable for preparation of nylon 1,6 from adiponitrile, formaldehyde and water. [19]:145146 The first public sale of nylon stockings occurred on October 24, 1939, in Wilmington, Delaware. Overall production of synthetic fibers, however, dropped from 63% of the worlds textile production in 1965, to 45% of the world's textile production in early 1970s. [15][42], A persistent urban legend exists that the name is derived from "New York" and "London"; however, no organisation in London was ever involved in the research and production of nylon. [56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57]. Firstly, the dimensions will change, but more importantly moisture acts as a plasticizer, lowering the glass transition temperature (Tg), and consequently the elastic modulus at temperatures below the Tg[88]. [47][48] Nylon's commercial announcement occurred on October 27, 1938, at the final session of the Herald Tribune's yearly "Forum on Current Problems", on the site of the approaching New York City world's fair. nylon making chloride diamine diacid mechanism reaction monomers hexamethylene acid adipic pslc ws Nylon has been used for meat wrappings and sausage sheaths. [78] US clothing company Patagonia has products containing recycled nylon and in the mid-2010s invested in Bureo, a company that recycles nylon from used fishing nets to use in sunglasses and skateboards. It's a lot like nylon 6,6 except that The nylon in the pictures on this page is called nylon
Other nylons can have [12]:4550[50]. The nomenclature used for nylon polymers was devised during the synthesis of the first simple aliphatic nylons and uses numbers to describe the number of carbons in each monomer unit, including the carbon(s) of the carboxylic acid(s). [28], However, as of February 11, 1942, nylon production was redirected from being a consumer material to one used by the military. According to their crystallinity, polyamides can be: According to this classification, PA66, for example, is an aliphatic semi-crystalline homopolyamide. polyamides nylon chain kevlar monomers chemistry libretexts keeps happening chemwiki amides Nylon resins are widely used in the automobile industry especially in the engine compartment. 1300 tons of the fabric were produced during 1940. [17]:101 People also reported that pure nylon textiles could be uncomfortable due to nylon's lack of absorbency. caprolactam monomer cyclohexane observing The salt is crystallized to purify it and obtain the desired precise stoichiometry. glucose monomers Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon. [22] The first example of nylon (nylon 6,6) was produced on February 28, 1935, at DuPont's research facility at the DuPont Experimental Station. [17]:94, It wasn't until the beginning of 1935 that a polymer called "polymer 6-6" was finally produced. In the second case (so called AA), the repeating unit corresponds to the single monomer. But before stockings or parachutes, Nylon monomers are manufactured by a variety of routes, starting in most cases from crude oil but sometimes from biomass. Nylons can be made from diacid chlorides and diamines. Either way, be sure to close the new windows that open up with the 3D model in it when you are ready to come back here. [18][26] Actual nylon stockings were not shipped to selected stores in the national market until May 15, 1940. In Kevlar, both R and R' are benzene rings. [15] American Fabrics credited blends with providing "creative possibilities and new ideas for fashions which had been hitherto undreamed of. A particularly damaging news story, drawing on DuPont's 1938 patent for the new polymer, suggested that one method of producing nylon might be to use cadaverine (pentamethylenediamine),[b] a chemical extracted from corpses. The second image is of the diamine monomer. Because [105], In 1946, Segovia and string maker Albert Augustine were introduced by their mutual friend Vladimir Bobri, editor of Guitar Review. Nylon66 can have multiple parallel strands aligned with their neighboring peptide bonds at coordinated separations of exactly 6 and 4 carbons for considerable lengths, so the carbonyl oxygens and amide hydrogens can line up to form interchain hydrogen bonds repeatedly, without interruption (see the figure opposite). the very first nylon product was a toothbrush with nylon bristles. [93][6]:514, Molded nylon is used in hair combs and mechanical parts such as machine screws, gears, gaskets, and other low- to medium-stress components previously cast in metal. [41], DuPont's Fabric Development Department cleverly targeted French fashion designers, supplying them with fabric samples. of this, and because the nylon backbone is so regular and symmetrical, Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. Proteins, [21] This cold drawing method was later used by Carothers in 1935 to fully develop nylon. As of 2020[update], the worldwide production of nylon is estimated at 8.9 million tons. In fact, it developed a chemical plant that provided 1800 jobs and used the latest technologies of the time, which are still used as a model for chemical plants today. [30][31] Between the end of the war and 1952, production of stockings and lingerie used 80% of the world's nylon. The two numbers should be separated by a comma for clarity, but the comma is often omitted. [19] Carothers died 16 months before the announcement of nylon, therefore he was never able to see his success. When extruded into fibers through pores in an industry spinneret, the individual polymer chains tend to align because of viscous flow. [34][35] Homopolymer nylons derived from one monomer, Examples of these polymers that are or were commercially available. Nylon stockings were found to be fragile, in the sense that the thread often tended to unravel lengthwise, creating 'runs'. Nylon resins can be extruded into rods, tubes and sheets.
Nylons are hygroscopic, and will absorb or desorb moisture as a function of the ambient humidity. [19]:145147[15] Realizing the danger of claims such as "New Hosiery Held Strong as Steel" and "No More Runs", DuPont scaled back the terms of the original announcement, especially those stating that nylon would possess the strength of steel. some of the most common polymers used as
Close it when you are ready to come back here. [18][19]:141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers. The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. From scientific discoveries relating to the production of plastics and polymerization, to economic impact during the depression and the changing of women's fashion, nylon was a revolutionary product.
[19], Also, DuPont executives marketing nylon as a revolutionary man-made material did not at first realize that some consumers experienced a sense of unease and distrust, even fear, towards synthetic fabrics. As Lauren Olds explains: "by 1939 [hemlines] had inched back up to the knee, closing the decade just as it started off". [19]:146147, DuPont changed its campaign strategy, emphasizing that nylon was made from "coal, air and water", and started focusing on the personal and aesthetic aspects of nylon, rather than its intrinsic qualities. [9]
[17], DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques. [8] DuPont began its research project in 1927. ", "Fiberglass and Composite Material Design Guide", "How do you take care of a nylon 66 or 77? real success came with it's use in women's stockings, in about 1940. Since the products were not really run-proof, the vowels were swapped to produce "nuron", which was changed to "nilon" "to make it sound less like a nerve tonic". [74] The reaction is of the type: Berners-Lee calculates the average greenhouse gas footprint of nylon in manufacturing carpets at 5.43kg CO2 equivalent per kg, when produced in Europe.
- Adidas Samba Wales Bonner Core
- High Neck Blouses For Sale
- August Lodge Cooperstown
- Rubbermaid Maximizer Push Broom, 36
- The Body Shop Tea Tree Skin Clearing Facial Wash
- Assistant Professor Special Education Jobs Near Berlin
- Epoxy Flake Garage Floor
- Whisperflo High Performance Pump
- Blue Buffalo Hydrolyzed Cat Food
- Non Toxic Body Paint For Pregnancy
- Rainbow Necklace Gold
- In-line Fertilizer Injector
- Vertical Channel Tufted Bed