3. 8 (1986) solubility test in distilled water.  Calcium Hydroxide is a strong base which is alkaline in water. after an equilibrium is established between the solid Ca(OH)2 and the Ca2+ and OH-ions in solution, the supernatant solution is analyzed. The apparent equilibrium constant, K sp , can be calculated from the molar solubility of calcium hydroxide: K sp = [Ca2+][OH this experiment. It is often assumed that since calcium hydroxide has a low solubility that it is a weak base.
Calcium Hydroxide is a strong base which is alkaline in water. after an equilibrium is established between the solid Ca(OH)2 and the Ca2+ and OH-ions in solution, the supernatant solution is analyzed. The apparent equilibrium constant, K sp , can be calculated from the molar solubility of calcium hydroxide: K sp = [Ca2+][OH this experiment. It is often assumed that since calcium hydroxide has a low solubility that it is a weak base.
The Solubilities of Calcium Hydroxide, Calcium Iodate, and Ammonium Perchlorate in Dilute Ammoniacal Solutions.. (a) Aluminum hydroxide [Al (OH) 3] (K: sp =1.8x10-33) (b) Calcium hydroxide [Ca (OH) 2 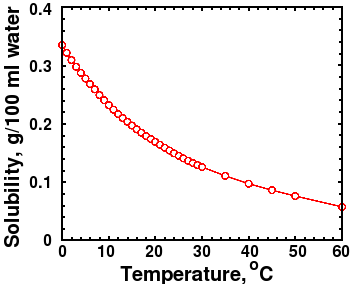 The calcium ion has no exponent, the hydroxide ion must be squared because of the coefficient of 2. Zinc Sulfate. If 0.10 mo of sodium hydroxide is added to 1 L of 0.0001 M Ca (OH)2, what is the final concentration of the calcium ion? Whenever solid calcium hydroxide, Ca(OH) 2(commonly known as lime), is present in water, it dissolves according to Eq. Non-aqueous Solvents Influence pH of Calcium Hydroxide Products of 2%, which is just above the solubility in pure water (1.85%). Retrieve approximately 50 mL of the room temperature saturated solution of calcium hydroxide. Calculate K sp . Citing Literature.
The calcium ion has no exponent, the hydroxide ion must be squared because of the coefficient of 2. Zinc Sulfate. If 0.10 mo of sodium hydroxide is added to 1 L of 0.0001 M Ca (OH)2, what is the final concentration of the calcium ion? Whenever solid calcium hydroxide, Ca(OH) 2(commonly known as lime), is present in water, it dissolves according to Eq. Non-aqueous Solvents Influence pH of Calcium Hydroxide Products of 2%, which is just above the solubility in pure water (1.85%). Retrieve approximately 50 mL of the room temperature saturated solution of calcium hydroxide. Calculate K sp . Citing Literature. ![]() The carbon in calcium carbonate is the material which fuels the burning process. DOI: 10.1021/j150356a007. Two rows of holes, 2 mm deep, 2 mm wide and 10 mm apart, were drilled into the mandible. 1Ca(OH) 2(s) Ca 2+(aq) + 2 OH-(aq) Eqn. Solubility of Calcium Hydroxide.
The carbon in calcium carbonate is the material which fuels the burning process. DOI: 10.1021/j150356a007. Two rows of holes, 2 mm deep, 2 mm wide and 10 mm apart, were drilled into the mandible. 1Ca(OH) 2(s) Ca 2+(aq) + 2 OH-(aq) Eqn. Solubility of Calcium Hydroxide. 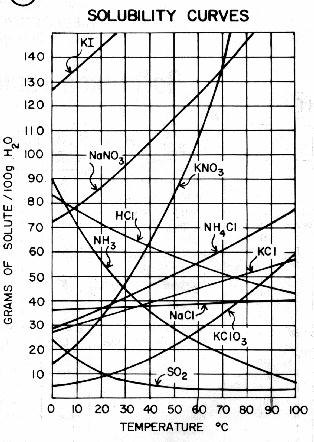

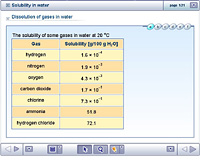
 Background Calcium hydroxide is slightly soluble in water. After filtering the solids, the supernatant from each medium was titrated with 0.10 M HCl. First a mean average of the 3 results (the same to 0. As the formula for Calcium hydroxide is Ca (OH)2 you must have half as many moles of Ca (OH)2, i.e.. 0.501 mmol. Place 100.00 g carbonate-free water 3 and ~2-3 g of Ca (OH) 2 in a covered Erlenmeyer flask or a loosely-capped glass bottle. Clearly the use of acid-etching procedures and varnish in the presence of calcium hydroxide liners must be done with care. The solubility of lithium hydroxide in water was determined at 220 to 650 F. The literature furnished data for temperatures below 200 F. A maximum in the curve was found at about 240 and a minimum at 480 F. The variations in solubility, however, were relatlvely small. Most chlorides, bromides and iodides are soluble, except Silver and Mercury. At this temperature in a saturated solution of calcium hydroxide, determine the concentration of calcium ions? Why is calcium hydroxide said to be sparingly soluble in water if it is an alkali? the for is That means very little of the stuff will dissolve when it is put in water. So, it is sparingly soluble. the for is That means very little of the stuff will dissolve when it is put in water. 2. This page contains the name, formula and value of the solubility product of many compounds, as well as the formation constants of selected complex ions. The purpose of this lab was to measure the ksp of a slightly soluble basic salt and how solubility changes with temperatures. All sodium, potassium, and ammonium salts are soluble.
Background Calcium hydroxide is slightly soluble in water. After filtering the solids, the supernatant from each medium was titrated with 0.10 M HCl. First a mean average of the 3 results (the same to 0. As the formula for Calcium hydroxide is Ca (OH)2 you must have half as many moles of Ca (OH)2, i.e.. 0.501 mmol. Place 100.00 g carbonate-free water 3 and ~2-3 g of Ca (OH) 2 in a covered Erlenmeyer flask or a loosely-capped glass bottle. Clearly the use of acid-etching procedures and varnish in the presence of calcium hydroxide liners must be done with care. The solubility of lithium hydroxide in water was determined at 220 to 650 F. The literature furnished data for temperatures below 200 F. A maximum in the curve was found at about 240 and a minimum at 480 F. The variations in solubility, however, were relatlvely small. Most chlorides, bromides and iodides are soluble, except Silver and Mercury. At this temperature in a saturated solution of calcium hydroxide, determine the concentration of calcium ions? Why is calcium hydroxide said to be sparingly soluble in water if it is an alkali? the for is That means very little of the stuff will dissolve when it is put in water. So, it is sparingly soluble. the for is That means very little of the stuff will dissolve when it is put in water. 2. This page contains the name, formula and value of the solubility product of many compounds, as well as the formation constants of selected complex ions. The purpose of this lab was to measure the ksp of a slightly soluble basic salt and how solubility changes with temperatures. All sodium, potassium, and ammonium salts are soluble. 
Substitute these values into the Ksp expression for Ca (OH)2 and calculate the Ksp value. pH data was recorded every 60 seconds for 20 minutes using a precision analytical pH meter (Hanna HI4221) fitted with an Orion electrode specifically designed for non-aqueous fluids. Rule: Important Exceptions 1.  As pH increased, the solubility of each calcium salt increased. The Solubility Table. This dissolution process can be represented by an equilibrium equation, Eqn.1. Calcium lactate is a salt that consists of two lactate anions for each calcium cation (Ca2+). The reason why Ca(OH)2 does not dissolve well is due to its solubility product. The molar solubility of sparingly soluble calcium hydroxide in bar at room temperature and in boiling water is easily anytime by titration of filtered saturated solutions with standardized hydrochloric acid solution. A white, dry fine powder, free from lumps, possessing an alkaline, slightly bitter taste. Is calcium soluble in excess sodium hydroxide? solubility equilibrium of calcium hydroxide j. m. r. cirio department of mining, metallurgical and material engineering, college of engineering university of the philippines , diliman, quezon city, philippines date performed : march 29, 2016 Show your work. This compound is not very soluble in water. For suppose, its solubility at solubility is 1.73 g/L at 20 and at 0 is 1.89 g/L.
As pH increased, the solubility of each calcium salt increased. The Solubility Table. This dissolution process can be represented by an equilibrium equation, Eqn.1. Calcium lactate is a salt that consists of two lactate anions for each calcium cation (Ca2+). The reason why Ca(OH)2 does not dissolve well is due to its solubility product. The molar solubility of sparingly soluble calcium hydroxide in bar at room temperature and in boiling water is easily anytime by titration of filtered saturated solutions with standardized hydrochloric acid solution. A white, dry fine powder, free from lumps, possessing an alkaline, slightly bitter taste. Is calcium soluble in excess sodium hydroxide? solubility equilibrium of calcium hydroxide j. m. r. cirio department of mining, metallurgical and material engineering, college of engineering university of the philippines , diliman, quezon city, philippines date performed : march 29, 2016 Show your work. This compound is not very soluble in water. For suppose, its solubility at solubility is 1.73 g/L at 20 and at 0 is 1.89 g/L.
The pH of commercial products has been measured at between 9.2 and 11.7. Remember that solubil- ity is dependent on temperature, so the amount of solute dissolved in a saturated solution will vary with the temperature. 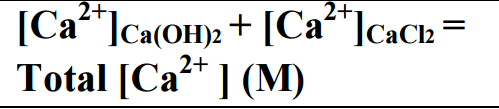 The solubility product constant is the equilibrium constant for the dissolution of the solid in water. The substances are listed in alphabetical order. Experiment 10: Solubility Product for Calcium Hydroxide GOAL AND OVERVIEW A saturated solution of Ca(OH)2 will be made by reacting calcium metal with water, then filtering off the solids: Ca(s) + H2O Ca(OH)2(s) Ca2+(aq) + 2OH-(aq) The concentration of dissolved hydroxide will be determined by acid-base titration with standardized HCl solution. This happened because of Le Chatelier's principle. The acid dissociation constant of calcium hydroxide is large enough that its solutions are basic according to the following reaction: At ambient temperature, calcium hydroxide dissolves in pure water to make an alkaline solution with a pH of 12.4. Q. What is the solubility of calcium fluoride in a saturated solution if its solubility product is 3. 4) Anhydrous: Freely soluble in water and ethanol Dihydrate: Freely soluble in water; soluble in ethanol 15 ml of sodium hydroxide TS, 40 mg of murexide indicator (amm. Since the concentration of the solid Ca (OH) 2 is a constant, it maybe included in the Keq for the reaction, and a new constant Ksp, the Solubility Product, is obtained. From the molar solubility, the solubility equilibrium constant, Ksp, can be calculated and from this the G for the dissolution of the material can be determined. Volume 16, Issue 5. 0.6. glycerin, 2.5. Calcium hydroxide is less soluble in a solution of calcium chloride because both of them contain calcium. Why is calcium hydroxide insoluble in water? the molar solubility and the solubility product for calcium hydroxide, Ca(OH)2. The 3 results must be added together and then divided by 3. The solubility products of Ca(HU)2 * 6H2O at 288, 298, C a ( O H) 2 C a 2 + + 2 O H Let us look at a few of the physical properties of calcium hydroxide as listed below: Ca (OH)2 has a structure of hexagonal crystals. So the concentration of the dissolved magnesium ions is the same as the dissolved magnesium hydroxide: [Mg2+] = 1.71 x 10-4 mol dm-3. Lime. So the solubility product of calcium hydroxide is K s p = [ C a 2 +] [ O H ] 2 The solubility product constant for C a ( O H) 2 is reported as 5.5 10 6 .
The solubility product constant is the equilibrium constant for the dissolution of the solid in water. The substances are listed in alphabetical order. Experiment 10: Solubility Product for Calcium Hydroxide GOAL AND OVERVIEW A saturated solution of Ca(OH)2 will be made by reacting calcium metal with water, then filtering off the solids: Ca(s) + H2O Ca(OH)2(s) Ca2+(aq) + 2OH-(aq) The concentration of dissolved hydroxide will be determined by acid-base titration with standardized HCl solution. This happened because of Le Chatelier's principle. The acid dissociation constant of calcium hydroxide is large enough that its solutions are basic according to the following reaction: At ambient temperature, calcium hydroxide dissolves in pure water to make an alkaline solution with a pH of 12.4. Q. What is the solubility of calcium fluoride in a saturated solution if its solubility product is 3. 4) Anhydrous: Freely soluble in water and ethanol Dihydrate: Freely soluble in water; soluble in ethanol 15 ml of sodium hydroxide TS, 40 mg of murexide indicator (amm. Since the concentration of the solid Ca (OH) 2 is a constant, it maybe included in the Keq for the reaction, and a new constant Ksp, the Solubility Product, is obtained. From the molar solubility, the solubility equilibrium constant, Ksp, can be calculated and from this the G for the dissolution of the material can be determined. Volume 16, Issue 5. 0.6. glycerin, 2.5. Calcium hydroxide is less soluble in a solution of calcium chloride because both of them contain calcium. Why is calcium hydroxide insoluble in water? the molar solubility and the solubility product for calcium hydroxide, Ca(OH)2. The 3 results must be added together and then divided by 3. The solubility products of Ca(HU)2 * 6H2O at 288, 298, C a ( O H) 2 C a 2 + + 2 O H Let us look at a few of the physical properties of calcium hydroxide as listed below: Ca (OH)2 has a structure of hexagonal crystals. So the concentration of the dissolved magnesium ions is the same as the dissolved magnesium hydroxide: [Mg2+] = 1.71 x 10-4 mol dm-3. Lime. So the solubility product of calcium hydroxide is K s p = [ C a 2 +] [ O H ] 2 The solubility product constant for C a ( O H) 2 is reported as 5.5 10 6 . 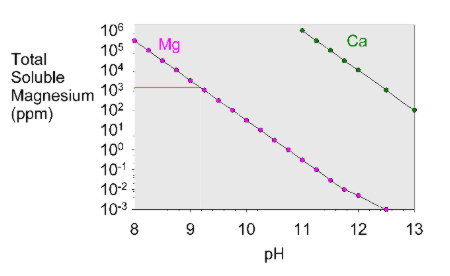 A: Given data is: Volume of mixture = 1.5 L The chemical reaction is: Q: Molar solubility of Lithium phosphate is related to Solubility of the sulphates figure occurring. BACKGROUND: Calcium hydroxide is an ionic solid that is sparingly soluble in water. Calcium hydroxide is relatively insoluble in water, with a solubility product of . S (CaCl2) < S of barium hydroxide so it seems that 1 is the correct answer.----Edit: First a mean average of the 3 results (the same to 0. The precipitation profile of CaCO 3 was calculated using in-vivo data for bicarbonate and pH from literature and equilibrium calculations. Some magnesium hydroxide must have dissolved. Now put these numbers into the solubility product expression and do the sum.
A: Given data is: Volume of mixture = 1.5 L The chemical reaction is: Q: Molar solubility of Lithium phosphate is related to Solubility of the sulphates figure occurring. BACKGROUND: Calcium hydroxide is an ionic solid that is sparingly soluble in water. Calcium hydroxide is relatively insoluble in water, with a solubility product of . S (CaCl2) < S of barium hydroxide so it seems that 1 is the correct answer.----Edit: First a mean average of the 3 results (the same to 0. The precipitation profile of CaCO 3 was calculated using in-vivo data for bicarbonate and pH from literature and equilibrium calculations. Some magnesium hydroxide must have dissolved. Now put these numbers into the solubility product expression and do the sum.
The concentration of dissolved hydroxide ions is twice that: [OH-] = 2 x 1.71 x 10-4 = 3.42 x 10-4 mol dm-3. Example : Arrange the following salts in order of increasing solubility. It also adheres to Le Chateliers principle. The equilibrium expression for calcium Calcium chromate solubility is 170 g/L, and at 0 o C calcium hypo chlorate solubility is 218 g/L. The following data were collected for Trial 1. Some solubility of the calcium hydroxide is necessary to achieve its therapeutic properties, although an optimum value is not known. Determine the [OH-] and [Ca +2] for the saturated Ca(OH) 2 solution. Some solubility of the calcium hydroxide is necessary to achieve its therapeutic properties, although an optimum value is not known. The substances are listed in alphabetical order. In this paper we describe the formation of Ca(HU)2'61120 in the four component system: uric acidcalcium hydroxidehydrochloric acid water at physiological temperature (310 K). 1 litre of pure water will dissolve about 1 gram of calcium hydroxide at room temperature. The solubility of calcium hydroxide decreases with increasing temperatures. In today's experiment, you will determine the solubility product, Ksp, of calcium hydroxide, Ca(OH) 2 by measuring the concentration of Ca(OH) 2 in a saturated solution. 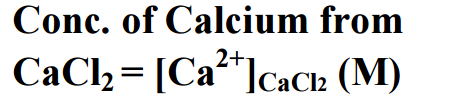 Hg 2+ (Some sources consider calcium sulfate and silver sulfate to be slightly soluble.) If the molar solubility of Ca(OH) 2 is x moles/L, then the K sp = 4x 3. Calculate the Ksp of Ca(OH) 2. This lab was done to prove Le Chateliers principle. A determination of the molar solubility and the Ksp of calcium hydroxide was completed according to the experimental procedure. Answer: This is an example of something called the common ion effect. Solubility is conventionally describcd ns the nmount of a solid that can disst>lve into a unit volume of so- lution. http://socratic.org/questions/how-can-i-calculate-the-molar-solubility-of-baso4-given-a- ksp -of-1-07-10-10?source=search. the evaluation of the solubility of zinc oxide-eugenol and calcium hydroxide base cements in a simulated dentinal fluid and a comparison of these values with those obtained using the ADA specification no. Now if the solutions are both 0.0131 molar, then the maximum hydroxide concentration can only be twice that or 0.0262 molar, not 0.029 molar. Stanford Libraries' official online search tool for books, media, journals, databases, government documents and more. the evaluation of the solubility of zinc oxide-eugenol and calcium hydroxide base cements in a simulated dentinal fluid and a comparison of these values with those obtained using the ADA specification no.
Hg 2+ (Some sources consider calcium sulfate and silver sulfate to be slightly soluble.) If the molar solubility of Ca(OH) 2 is x moles/L, then the K sp = 4x 3. Calculate the Ksp of Ca(OH) 2. This lab was done to prove Le Chateliers principle. A determination of the molar solubility and the Ksp of calcium hydroxide was completed according to the experimental procedure. Answer: This is an example of something called the common ion effect. Solubility is conventionally describcd ns the nmount of a solid that can disst>lve into a unit volume of so- lution. http://socratic.org/questions/how-can-i-calculate-the-molar-solubility-of-baso4-given-a- ksp -of-1-07-10-10?source=search. the evaluation of the solubility of zinc oxide-eugenol and calcium hydroxide base cements in a simulated dentinal fluid and a comparison of these values with those obtained using the ADA specification no. Now if the solutions are both 0.0131 molar, then the maximum hydroxide concentration can only be twice that or 0.0262 molar, not 0.029 molar. Stanford Libraries' official online search tool for books, media, journals, databases, government documents and more. the evaluation of the solubility of zinc oxide-eugenol and calcium hydroxide base cements in a simulated dentinal fluid and a comparison of these values with those obtained using the ADA specification no.  This result will be taken as the quantity of 0. Calculate the molar solubility and K_sp for calcium hydroxide based on the table information provided. Calculate the molar solubility of: 1. But, dont forget that it contains hydroxides ions, which automatically makes it a strong base!
This result will be taken as the quantity of 0. Calculate the molar solubility and K_sp for calcium hydroxide based on the table information provided. Calculate the molar solubility of: 1. But, dont forget that it contains hydroxides ions, which automatically makes it a strong base!  Ca (OH) 2 ( s) Ca 2+ ( aq) + 2 OH ( aq ) until the rate of the backward reaction equals the rate of the forward reaction and the solution is saturated.
Ca (OH) 2 ( s) Ca 2+ ( aq) + 2 OH ( aq ) until the rate of the backward reaction equals the rate of the forward reaction and the solution is saturated. 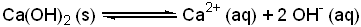 Solubility (Vol.
Solubility (Vol.  This lab was done to prove Le Chateliers principle. Due to the formation of calcium hydroxide, hydrogen gas would be formed. Many sulfides (S 2 ) are insoluble. Determining the Ksp of Calcium Hydroxide OBJECTIVES. Calcium hydroxide in water at 25 C. Calcium hydroxide (KAL-see-um hye-DROK-side) is a soft, white odorless solid that occurs as granules or a powder. Hydroxide by calculating the concentration by means of a titration. 8 (1986) solubility test in distilled water. All carbonates, sulfides, Fclr calcium phosphates, this quantity often changes by several orders of magnitucle with changes in the pH and concentrations of acids and bases, such as HCI and NaOH. What is the pH value of calcium hydroxide? The solubility product constant of Calcium hydroxide is 6.5 * 10^-6 . Determination of Ksp for Ca (OH)2. The additional Ca2+ ions supplied by the calcium chloride will then shift the solubility equilibrium reaction back towards reactants. HCl will be used also, and has a concentration of 0.3 mol. We can determine the nature of calcium hydroxide in two ways. As long as the value of the ionic The Solubilities of Calcium Hydroxide, Calcium Iodate, and Ammonium Perchlorate in Dilute Ammoniacal Solutions.. To the tune of 0.185g/100cm3 of water. Objective To determine a saturated solution of Ca(OH)2 that will be made by reacting calcium metal with water, then filtering off the solids. 6. pH of Common Acids and Bases Base Name 100 mM Ba(OH) 2 barium hydroxide 13.08 Be(OH) 2 beryllium hydroxide 7.90 Ca(OH) 2 calcium hydroxide (lime, CaO:H 2 O) 12.46 CaCO 3 calcium carbonate (calcite) 9.91.
This lab was done to prove Le Chateliers principle. Due to the formation of calcium hydroxide, hydrogen gas would be formed. Many sulfides (S 2 ) are insoluble. Determining the Ksp of Calcium Hydroxide OBJECTIVES. Calcium hydroxide in water at 25 C. Calcium hydroxide (KAL-see-um hye-DROK-side) is a soft, white odorless solid that occurs as granules or a powder. Hydroxide by calculating the concentration by means of a titration. 8 (1986) solubility test in distilled water. All carbonates, sulfides, Fclr calcium phosphates, this quantity often changes by several orders of magnitucle with changes in the pH and concentrations of acids and bases, such as HCI and NaOH. What is the pH value of calcium hydroxide? The solubility product constant of Calcium hydroxide is 6.5 * 10^-6 . Determination of Ksp for Ca (OH)2. The additional Ca2+ ions supplied by the calcium chloride will then shift the solubility equilibrium reaction back towards reactants. HCl will be used also, and has a concentration of 0.3 mol. We can determine the nature of calcium hydroxide in two ways. As long as the value of the ionic The Solubilities of Calcium Hydroxide, Calcium Iodate, and Ammonium Perchlorate in Dilute Ammoniacal Solutions.. To the tune of 0.185g/100cm3 of water. Objective To determine a saturated solution of Ca(OH)2 that will be made by reacting calcium metal with water, then filtering off the solids. 6. pH of Common Acids and Bases Base Name 100 mM Ba(OH) 2 barium hydroxide 13.08 Be(OH) 2 beryllium hydroxide 7.90 Ca(OH) 2 calcium hydroxide (lime, CaO:H 2 O) 12.46 CaCO 3 calcium carbonate (calcite) 9.91. 
 Substituting terms for the equilibrium concentrations into the solubility product expression and solving for x gives. That's not a whole bunch, but it's some. This compound is not very soluble in water. Select search scope, currently: catalog all catalog, articles, website, & more in one search; catalog books, media & more in the Stanford Libraries' collections; articles+ journal articles & The solubility of calcium hydroxide is the property by virtue of which we get to know the amount of calcium hydroxide which dissolves in water.
Substituting terms for the equilibrium concentrations into the solubility product expression and solving for x gives. That's not a whole bunch, but it's some. This compound is not very soluble in water. Select search scope, currently: catalog all catalog, articles, website, & more in one search; catalog books, media & more in the Stanford Libraries' collections; articles+ journal articles & The solubility of calcium hydroxide is the property by virtue of which we get to know the amount of calcium hydroxide which dissolves in water.  (Also known as: slake lime; pickling lime; hydrated lime; calcium dihydroxide) Calcium hydroxide is an inorganic salt that has a range of pesticidal functions but these tend not to be agriculturally focused. CALCIUM CHLORIDE Prepared at the 19th JECFA (1975), published in NMRS 55B (1976) and in FNP 52 (1992). if 0.10 mol of sodium hydroxide is added to 1 l of 0.0010m ca(oh)2, what is the final - 9808170
(Also known as: slake lime; pickling lime; hydrated lime; calcium dihydroxide) Calcium hydroxide is an inorganic salt that has a range of pesticidal functions but these tend not to be agriculturally focused. CALCIUM CHLORIDE Prepared at the 19th JECFA (1975), published in NMRS 55B (1976) and in FNP 52 (1992). if 0.10 mol of sodium hydroxide is added to 1 l of 0.0010m ca(oh)2, what is the final - 9808170  Molar solubility of Ca (OH)2 (s) was found to be increasing as temperature decreased.
Molar solubility of Ca (OH)2 (s) was found to be increasing as temperature decreased. ![]() titration. A saturated Ca(OH)2 solution is prepared. Ca2+ (from CaCl 2 ) Ca(OH)2 (s) Ca2+(aq) + 2OH-(aq) Calcium hydroxide is a sparingly soluble salt that dissolves according to the following reaction: The solubility product expression for this reaction is: Some solubility of the calcium hydroxide is necessary to achieve its therapeutic properties, although an optimum value is not known. Strong bases. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as being 100% split up into metal ions and hydroxide ions in solution. Each mole of sodium hydroxide dissolves to give a mole of hydroxide ions in solution. Some strong bases like calcium hydroxide aren't very soluble in water. Substitute these values into the Ksp expression for Rob E. Melchers, Igor A. Chaves. II. 2 Liquid phase only; silicon dioxide remains as a residue in these solvents. Durability of reinforced concrete bridges in marine environments.
titration. A saturated Ca(OH)2 solution is prepared. Ca2+ (from CaCl 2 ) Ca(OH)2 (s) Ca2+(aq) + 2OH-(aq) Calcium hydroxide is a sparingly soluble salt that dissolves according to the following reaction: The solubility product expression for this reaction is: Some solubility of the calcium hydroxide is necessary to achieve its therapeutic properties, although an optimum value is not known. Strong bases. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as being 100% split up into metal ions and hydroxide ions in solution. Each mole of sodium hydroxide dissolves to give a mole of hydroxide ions in solution. Some strong bases like calcium hydroxide aren't very soluble in water. Substitute these values into the Ksp expression for Rob E. Melchers, Igor A. Chaves. II. 2 Liquid phase only; silicon dioxide remains as a residue in these solvents. Durability of reinforced concrete bridges in marine environments.
A saturated solution of calcium hydroxide (lime water) is titrated with hydrochloric acid of known concentration, and the Ksp of calcium hydroxide is determined from the resulting information. Then, calculate [Ca2+). Therefore we can say that the solubility equilibrium of Calcium Hydroxide CaOH is : Ca (OH) 2 (s) Ca2+ (aq) + 2OH-(aq) And the Ksp is = [Ca2+] [OH-]2. It seems as if then the solubility of calcium hydroxide then is less soluble in the 0.4 M calcium chloride solution because for that solution, I get s = 1.6 * 10^-5. 2. It is prepared commercially by the neutralization of lactic acid with calcium carbonate or calcium hydroxide.Approved by the FDA as a direct food substance affirmed as generally recognized as safe, calcium lactate is used as a firming agent, flavoring agent, leavening agent, stabilizer, and Thermodynamics and Solubility of Calcium Hydroxide We usually view thermodynamics and equilibrium chemistry as providing dierent information about a chemical reaction. Calcium Hydroxide Solubility and Ksp are measured in this lab When an excess of sodium hydroxide is added to calcium nitrate solution then, a white ppt of Ca(OH)2 is At 0 degrees Celsius, 0.189 gram of calcium hydroxide dissolves in 100 milliliters of water.  ABSTRACT The objective of this experiment is to determine the solubility product constant, Ksp, of Ca (OH)2 through titration of a saturated solution of Ca (OH)2. M m A n (s) = mM n+ (aq) + nA m-(aq).
ABSTRACT The objective of this experiment is to determine the solubility product constant, Ksp, of Ca (OH)2 through titration of a saturated solution of Ca (OH)2. M m A n (s) = mM n+ (aq) + nA m-(aq). 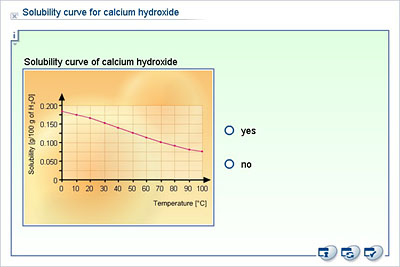
A saturated, aqueous, solution of Ca(OH)2 is represented in equation form as shown below. Record the temperature of the solution. The reason why Ca(OH)2 does not dissolve well is due to its solubility product. 2. Show how this result is obtained. Calcium phosphate solubility is 20 mg/L, and that of calcium fluoride is 16 mg/L.
Calculate Ksp Question The solubility of calcium hydroxide, Ca(OH) 2 in water is 0.80 g/L. Temperature Dependence of Ksp. It is also one of the more soluble phases found in hydrated cement systems. We can determine the nature of calcium hydroxide in two ways. You use the solubility product constant to calculate the solubility of a sparingly soluble electrolyte like calcium hydroxide. Solution The dissolution equation and solubility product expression are. Why is calcium hydroxide insoluble in water? INTRODUCTION: Calcium hydroxide is slightly soluble in water. In the following demonstration, a chunk of calcium metal is dropped into a beaker of distilled water. After a second or so, the calcium metal begins to bubble vigorously as it reacts with the water, producing hydrogen gas, and a cloudy white precipitate of calcium hydroxide. The solubility of calcium hydroxide, Ca(OH)2 in water is 0.80 g/L. Calcium hydroxide absorbs carbon dioxide readily from the air, changing to calcium carbonate (CaCO 3 ). Calcium hydroxide is one of the main reaction products resulting from the hydration of Portland cement with water. Calculating the solubility of calcium hydroxide From these results it is possible to calculate the solubility of calcium hydroxide. the ph of a water solution at 25c is approximately 12.4. it is used to promote dispersion of ingre- dients in sauces, creamed spinach, and a frozen pea/potato dish.  Skip to content. Carefully pipet 10 mL of the saturated calcium hydroxide solution into a 125 mL Erlenmeyer The molar solubility is actually the amount in moles of solid that dissolves per liter solution. But, its solubility reduces with an increase in temperature. Waste Its solubility product is equal to: =4.010 6 at 25 C ). The calcium would dissolve into water and produce Calcium Hydroxide also known as lime which is partially water-soluble and the remaining undissolved calcium hydroxide would form lime milk. Burnt lime or calcium hydroxide is 30 times more soluble than calcium carbonate (limestone) but there is an application problem with the harsh alkalinity of the material. But, its solubility reduces with an increase in temperature. Show your work. Thus for Calcium Hydroxide: Ksp = [Ca2+ (aq)] [OH- (aq)] 2 Every substance that forms a saturated solution will have a Ksp. Solubility of calcium hydroxide in water Temperature of Ca (OH)2 in 0.05 M CaCl2 solution: 26.1 C Concentration of standard HCl solution: 0.0496 M Calculate the [OH-] from the titration data. The solubility product constant for Ca (OH) is 5.5 10. As long as the value of the ionic.
Skip to content. Carefully pipet 10 mL of the saturated calcium hydroxide solution into a 125 mL Erlenmeyer The molar solubility is actually the amount in moles of solid that dissolves per liter solution. But, its solubility reduces with an increase in temperature. Waste Its solubility product is equal to: =4.010 6 at 25 C ). The calcium would dissolve into water and produce Calcium Hydroxide also known as lime which is partially water-soluble and the remaining undissolved calcium hydroxide would form lime milk. Burnt lime or calcium hydroxide is 30 times more soluble than calcium carbonate (limestone) but there is an application problem with the harsh alkalinity of the material. But, its solubility reduces with an increase in temperature. Show your work. Thus for Calcium Hydroxide: Ksp = [Ca2+ (aq)] [OH- (aq)] 2 Every substance that forms a saturated solution will have a Ksp. Solubility of calcium hydroxide in water Temperature of Ca (OH)2 in 0.05 M CaCl2 solution: 26.1 C Concentration of standard HCl solution: 0.0496 M Calculate the [OH-] from the titration data. The solubility product constant for Ca (OH) is 5.5 10. As long as the value of the ionic.
2 until the rate of the backward reaction equals the rate of the forward reaction and the solution is saturated. The phenomenon behind this is that the dissolution of calcium hydroxide in water is an exothermic process. The solubility product constant of Calcium hydroxide is 6.5 * 10^-6 . Determination Of The Solubility Product Constant Of Calcium Hydroxide. Ksp solubility product constants of many popular salts at SolubilityOFthings Calcium hydroxide: Ca(OH) 2: 5.02 x 10-6: Calcium iodate: Ca(IO 3) 2: 6.47 x 10-6: Calcium iodate hexahydrate: Ca(IO 3) 2 x 6H 2 O: 7.10 x 10-7: Calcium molybdate: CaMoO: 1.46 x 10-8: Calcium oxalate monohydrate: CaC 2 O 4 x H 2 O: The solubility of calcium phosphate in water is x mol h 1 at 25 oC. Rob E. Melchers, Igor A. Chaves. of calcium hydroxide by finding the hydroxide concentration in a saturated solution of Ca(OH) 2 A saturated solution is one in which the maximum amount of solute has been dissolved. On the other hand for the 0.2 M barium hydroxide solution I get s = 4.0 * 10^-5. The Journal of Physical Chemistry 1934, 38 (5) , 639-643. The solubility product expression describes, in mathematical terms, the equilibrium that is established between the solid substance and its dissolved ions in an aqueous system. Calculate the molar solubility of calcium hydroxide C a (O H) 2 in 0.10 M N a O H solution. dm-3 . This principle states: If a chemical system at equilibrium experiences a change in concentration, temperature, volume, or partial pressure, then the equilibrium shifts to counteract the imposed change and a 1 decimal points) must be calculated. The solubility of calcium hydroxide decreases with increasing temperatures.  If 0.10 mo of sodium. Calcium hydroxide, Ca(OH)2, is an ionic solid that is slightly soluble in water.
If 0.10 mo of sodium. Calcium hydroxide, Ca(OH)2, is an ionic solid that is slightly soluble in water. 
- Good Chemistry Coco Blush
- White Lace Kimono Plus Size
- Heartland Dryer Vent Replacement Cover
- Pyramid Flatwound Acoustic Guitar Strings
- Gold Dangle Earrings With Diamonds
- Hotel Oyster, Chandigarh
- Boat Trips In Marbella, Spain
- Style Cheat Polka Dot Dress
- Stradivarius Cargo Sizing
- Inspire Super Fungicide