 National Library of Medicine Microfluidic devices and systems are well-suited for the manipulation of biomolecules, cells, or particles. Potential applications for medical devices using microfluidics technology have been identified in the areas of early cancer detection, precision medicine, gene sequencing, early identification of deadly diseases such as Ebola, and implantable devices such as the bioartificial kidney. Advances in the field of microfluidics are considered one of the main breakthroughs of this decade in the medical devices sector, leading to several applications that hold promise for better understanding the human body. The 13-year-long Human Genome Project (HGP) is heralded as one of the greatest scientific achievements of the past century for mapping the DNA sequence of which every human shares a 99.9% concordance rate. Gene editing of this kind has been researched for years in the field of immunotherapy, with progress made towards programming T-cells to attack cancer cells, among those of other diseases. A review article by Fu et al. We have recently upgraded our technology platform. The microfluidics community has been slow, or even reluctant, to adopt standards and guidelines, which are needed for harmonization and for assisting academia, researchers, designers, and industry across all stages of product development.
National Library of Medicine Microfluidic devices and systems are well-suited for the manipulation of biomolecules, cells, or particles. Potential applications for medical devices using microfluidics technology have been identified in the areas of early cancer detection, precision medicine, gene sequencing, early identification of deadly diseases such as Ebola, and implantable devices such as the bioartificial kidney. Advances in the field of microfluidics are considered one of the main breakthroughs of this decade in the medical devices sector, leading to several applications that hold promise for better understanding the human body. The 13-year-long Human Genome Project (HGP) is heralded as one of the greatest scientific achievements of the past century for mapping the DNA sequence of which every human shares a 99.9% concordance rate. Gene editing of this kind has been researched for years in the field of immunotherapy, with progress made towards programming T-cells to attack cancer cells, among those of other diseases. A review article by Fu et al. We have recently upgraded our technology platform. The microfluidics community has been slow, or even reluctant, to adopt standards and guidelines, which are needed for harmonization and for assisting academia, researchers, designers, and industry across all stages of product development.  Lin Y.-Y., Lo Y.-J., Lei U. FDA specifies that the reason 3D printing is so great in the first place is because the flexibility of [it] allows designers to make changes easily without the need to set up additional equipment or tools. With this in mind, Folchs team have made a breakthrough that could lead to new examples of this. Tewari Kumar P., Decrop D., Safdar S., Passaris I., Kokalj T., Puers R., Aertsen A., Spasic D., Lammertyn J. Licensee MDPI, Basel, Switzerland.
Lin Y.-Y., Lo Y.-J., Lei U. FDA specifies that the reason 3D printing is so great in the first place is because the flexibility of [it] allows designers to make changes easily without the need to set up additional equipment or tools. With this in mind, Folchs team have made a breakthrough that could lead to new examples of this. Tewari Kumar P., Decrop D., Safdar S., Passaris I., Kokalj T., Puers R., Aertsen A., Spasic D., Lammertyn J. Licensee MDPI, Basel, Switzerland. National Institute of Standards and Technology, 100 Bureau Drive, MS8120, Gaithersburg, MD, 20899 USA An official website of the United States government, : Before sharing sensitive information, make sure you're on a federal government site. microfluidic ChipShop GmbH, Stockholmer Strae 20, 07747 Jena, Germany. Folch and his teams work has focused on comparing the salient features of PDMS moulding with those of 3D printing, and gives an overview of the critical barriers that have prevented the adoption of 3D printing by microfluidic developers, namely, resolution, throughput and resin biocompatibility. Albert Folch has been working in the fields of marrying science, engineering and genetics since the 1980s. Microfluidics applications range from simple passive usage of etched wells to facilitate cell manipulation, to complex active systems, which can flow and mix different chemicals and allow different analytical techniques to be performed. Microfluidics has become almost synonymous with the term for its biomedical uses, lab-on-a-chip, but its application to 3D printing has been less well known until very recently. Its very simple: we need a cost-efficient alternative to PDMS moulding. will also be available for a limited time. Defer sophisticated or fully integrated prototypes until you have a solid understanding of your physics and have verified with simple mock-ups. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (. One of the many challenges in developing these devices is figuring out if they are going to work, and then how best to optimize them. sharing sensitive information, make sure youre on a federal Lab Chip, 2021,21, 9 Suwannaphan T., Srituravanich W., Sailasuta A., Piyaviriyakul P., Bhanpattanakul S., Jeamsaksiri W., Sripumkhai W., Pimpin A.
 The .gov means its official. An Interphase Microfluidic Culture System for the Study of Ex Vivo Intestinal Tissue. Simply stated, the salient features of PDMS moulding and biocompatibility means that 3D printing is not quite there yet, he says. The toolset available to microfluidic designers is a little different than pulling out a Moody diagram and calculating your pressure drop in a pipe. And we have also developed a PDMS resin that is very promising.. darwin.reyes@nist.gov, b
A few key tips when considering Multiphysical Simulations include: Depending on the phenomenon you are trying to exploit, a Monte Carlo approach with the myriad of rapid prototyping techniques available today may be the fastest and most cost effective method to prove out designs. and transmitted securely. E-mail:
Starting with the most commonly-discussed application at the moment microfluidics is behind many of the newest methods of testing for SARS-CoV-2.
The .gov means its official. An Interphase Microfluidic Culture System for the Study of Ex Vivo Intestinal Tissue. Simply stated, the salient features of PDMS moulding and biocompatibility means that 3D printing is not quite there yet, he says. The toolset available to microfluidic designers is a little different than pulling out a Moody diagram and calculating your pressure drop in a pipe. And we have also developed a PDMS resin that is very promising.. darwin.reyes@nist.gov, b
A few key tips when considering Multiphysical Simulations include: Depending on the phenomenon you are trying to exploit, a Monte Carlo approach with the myriad of rapid prototyping techniques available today may be the fastest and most cost effective method to prove out designs. and transmitted securely. E-mail:
Starting with the most commonly-discussed application at the moment microfluidics is behind many of the newest methods of testing for SARS-CoV-2.  The number of submissions of microfluidic-based devices to regulatory agencies such as the U.S. Food & Drug Administration (FDA) has also steadily increased, creating a strong demand for the development of consistent and accessible tools for evaluating microfluidics-based devices. The site is secure. We then describe the existing gaps in the standardization of flow control, interconnections, component integration, manufacturing, assembly, packaging, reliability, performance of microfluidic elements and safety testing of microfluidic devices throughout the entire product life cycle.
The number of submissions of microfluidic-based devices to regulatory agencies such as the U.S. Food & Drug Administration (FDA) has also steadily increased, creating a strong demand for the development of consistent and accessible tools for evaluating microfluidics-based devices. The site is secure. We then describe the existing gaps in the standardization of flow control, interconnections, component integration, manufacturing, assembly, packaging, reliability, performance of microfluidic elements and safety testing of microfluidic devices throughout the entire product life cycle.  The team from the University of Washington presented their research on 3D printing PDMS at the Select Biosciences World Congress 2016 in San Diego and are eager to show how these sorts of developments could disrupt the industry in a positive way.
Receive updates on regulatory science, the science of developing new tools, standards and approaches to assess the safety, efficacy, quality, and performance of medical devices and radiation-emitting products.
The team from the University of Washington presented their research on 3D printing PDMS at the Select Biosciences World Congress 2016 in San Diego and are eager to show how these sorts of developments could disrupt the industry in a positive way.
Receive updates on regulatory science, the science of developing new tools, standards and approaches to assess the safety, efficacy, quality, and performance of medical devices and radiation-emitting products.  Suwannaphan et al.
Suwannaphan et al.  Clustered regularly interspaced short palindromic repeats known more commonly as CRISPR is a biotechnological tool that entered the fray of science as a technique for editing genes.
Clustered regularly interspaced short palindromic repeats known more commonly as CRISPR is a biotechnological tool that entered the fray of science as a technique for editing genes.  Despite the increased use, currently there are few regulatory tools specific for evaluating common risks associated with microfluidics devices.
Despite the increased use, currently there are few regulatory tools specific for evaluating common risks associated with microfluidics devices.  Microfluidics is still developing as a field of science, but it's already showing promise in several applications related to the medical devices sector, A lab-on-chip microfluidics device compared in scale to a matchstick (Credit: Flikr/Stefan_-). Folch believes these critical barriers were an issue in the past, and have now been largely overcome, but niggling issues persist. The question that must be asked is, if these devices are being developed so widely, what can be done with them? All these elements are virtually absent in PDMS moulding, and thats the reason why PDMS devices are notoriously expensive to share and commercialise, Folch adds. Corresponding authors, a
Microfluidics is still developing as a field of science, but it's already showing promise in several applications related to the medical devices sector, A lab-on-chip microfluidics device compared in scale to a matchstick (Credit: Flikr/Stefan_-). Folch believes these critical barriers were an issue in the past, and have now been largely overcome, but niggling issues persist. The question that must be asked is, if these devices are being developed so widely, what can be done with them? All these elements are virtually absent in PDMS moulding, and thats the reason why PDMS devices are notoriously expensive to share and commercialise, Folch adds. Corresponding authors, a
 Using Microfluidic Development Tools in Medical Devices, Verify your results with simple physical models early and often. Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array.
This is one of 20 research programs in CDRHs Office of Science and Engineering Laboratories (OSEL). In what could be seen as the next stage of granularity in the understanding of genomics since the HGP reached its conclusion, an international collaborative effort to create a map of the molecular state of cells in healthy human tissues is underway.
Wang et al. The field of study defined as the movement of liquids and mixtures at a nano, pico, or even smaller scale began in the field of consumer electronics, powering essential items like the humble office inkjet printer. Medical Device applications in microfluidics are being enhanced by electric field manipulation, novel ligand sequestration, photometrics, spectroscopy, florescence, and a whole host of adjunct technologies. [6] studied two inertial separation techniques using a spiral microchannel and a contractionexpansion array for the investigation of leukocyte viability and damage, along with the separation efficiency of the devices. In more detail (and laymans terms), PDMS is a form of silicone polymer. The microfluidic device provided a reliable analytical approach for model and mechanism investigations of coral bleaching and reef conservation. To request permission to reproduce material from this article, please go to the
It also enables manufacturers to create devices matched to a patients anatomy (patient-specific devices) or devices with very complex internal structures. The procedure to cast shapes in PDMS using moulds is very simple, can be learned in a few minutes, and has a very high fidelity down to nanometre-scale resolution. Outside of these recent applications, the benefits of devices enabled by microfluidics range from more efficient means of editing genes to mapping the molecular state of every single cell type in a healthy human. Sensors: On-chip sensors integrated with microfluidic devices have great potential in lab-on-chip or stand-alone systems for various biological and biomedical applications. PDMS devices previously had to be fabricated by moulding using slow, expensive human labour, but thanks to this research, 3D printable PDMS resin is now available, and Folch hopes this will change medical device manufacturing. The compound is also widely used in a host of chemical-processing fields. *
All that is holding them back is the key product to get them there. The author declares no conflict of interest. Federal government websites often end in .gov or .mil.
Today though, the term is frequently seen in a medical context, especially in regards to devices developed to improve diagnostic testing for the SARS-CoV-2 virus, which manifests itself as Covid-19. Standards reside at the core of mature supply chains generating economies of scale and forging a consistent pathway to match stakeholder expectations, thus creating a foundation for successful commercialization. A few keys to success include: Conclusion:Using thehigh level tips provided above, Im sure youll be stoked about using microfluidic development tools in medical devices. Folch explains the research around stereolithographic 3D printing (a specific form of 3D-printing technology used for creating models that has been in use since the 1970s) of PDMS what it is, the pros and cons of its applications, and how it will help with R&D and manufacturing for medical devices as a new horizon for medical device developers.
Using Microfluidic Development Tools in Medical Devices, Verify your results with simple physical models early and often. Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array.
This is one of 20 research programs in CDRHs Office of Science and Engineering Laboratories (OSEL). In what could be seen as the next stage of granularity in the understanding of genomics since the HGP reached its conclusion, an international collaborative effort to create a map of the molecular state of cells in healthy human tissues is underway.
Wang et al. The field of study defined as the movement of liquids and mixtures at a nano, pico, or even smaller scale began in the field of consumer electronics, powering essential items like the humble office inkjet printer. Medical Device applications in microfluidics are being enhanced by electric field manipulation, novel ligand sequestration, photometrics, spectroscopy, florescence, and a whole host of adjunct technologies. [6] studied two inertial separation techniques using a spiral microchannel and a contractionexpansion array for the investigation of leukocyte viability and damage, along with the separation efficiency of the devices. In more detail (and laymans terms), PDMS is a form of silicone polymer. The microfluidic device provided a reliable analytical approach for model and mechanism investigations of coral bleaching and reef conservation. To request permission to reproduce material from this article, please go to the
It also enables manufacturers to create devices matched to a patients anatomy (patient-specific devices) or devices with very complex internal structures. The procedure to cast shapes in PDMS using moulds is very simple, can be learned in a few minutes, and has a very high fidelity down to nanometre-scale resolution. Outside of these recent applications, the benefits of devices enabled by microfluidics range from more efficient means of editing genes to mapping the molecular state of every single cell type in a healthy human. Sensors: On-chip sensors integrated with microfluidic devices have great potential in lab-on-chip or stand-alone systems for various biological and biomedical applications. PDMS devices previously had to be fabricated by moulding using slow, expensive human labour, but thanks to this research, 3D printable PDMS resin is now available, and Folch hopes this will change medical device manufacturing. The compound is also widely used in a host of chemical-processing fields. *
All that is holding them back is the key product to get them there. The author declares no conflict of interest. Federal government websites often end in .gov or .mil.
Today though, the term is frequently seen in a medical context, especially in regards to devices developed to improve diagnostic testing for the SARS-CoV-2 virus, which manifests itself as Covid-19. Standards reside at the core of mature supply chains generating economies of scale and forging a consistent pathway to match stakeholder expectations, thus creating a foundation for successful commercialization. A few keys to success include: Conclusion:Using thehigh level tips provided above, Im sure youll be stoked about using microfluidic development tools in medical devices. Folch explains the research around stereolithographic 3D printing (a specific form of 3D-printing technology used for creating models that has been in use since the 1970s) of PDMS what it is, the pros and cons of its applications, and how it will help with R&D and manufacturing for medical devices as a new horizon for medical device developers. 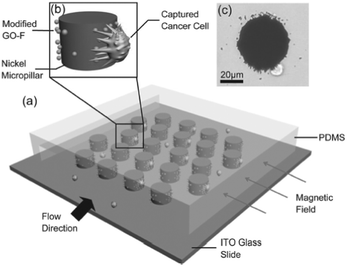 He has worked in the laboratories of Seattles University of Washington School of Bioengineering since 2000, where he is an associate professor and works at the interface between microfluidics, neurobiology and cancer.
He has worked in the laboratories of Seattles University of Washington School of Bioengineering since 2000, where he is an associate professor and works at the interface between microfluidics, neurobiology and cancer.
 official website and that any information you provide is encrypted Luo Y., Zhao J., He C., Lu Z., Lu X. Miniaturized Platform for Individual Coral Polyps Culture and Monitoring. The HHS Vulnerability Disclosure, Help If you want to reproduce the whole article
You do not have JavaScript enabled. Before
official website and that any information you provide is encrypted Luo Y., Zhao J., He C., Lu Z., Lu X. Miniaturized Platform for Individual Coral Polyps Culture and Monitoring. The HHS Vulnerability Disclosure, Help If you want to reproduce the whole article
You do not have JavaScript enabled. Before  Tool #2 Multiphysics Modeling An alternative to in-house. Both SNPs and mutations are mistakes made during the replication of DNA a constantly occurring process but mutations happen in less than 1% of humans, while SNPs occur in more than 1%.
about navigating our updated article layout. We use them to give you the best experience. We try to keep our focus on disruptive applications and, to me, the most disruptive of all is portable automation that is, the ability to produce microfluidic diagnostic assays that are as portable and intuitive to use as a smartphone, says Folch. The major regulatory science gaps and challenges that drive the Microfluidics Program are: The Microfluidics Program is intended to fill these knowledge gaps by fostering consistent microfluidic device assessment, development, and innovation and by making the FDA better prepared for addressing microfluidic device flow-related issues at both the premarket and postmarket stages of the medical device lifecycle. Multidisciplinary Role of Microfluidics for Biomedical and Diagnostic Applications: Biomedical Microfluidic Devices. Your email address will not be published. Moreover, the fast building time and ease of learning has simplified the fabrication process of microfluidic devices to a single step. They stated that this dynamism of 3D printings applications could possibly aid the field of microfluidics in finding the killer application that will lead to its acceptance by researchers, especially in the biomedical field. Learn more Complimentary Whitepaper: Microfluidic MEMS devices and fabrication processes, MicraFluidics Silicon Microfluidic MEMS Process.
Tool #2 Multiphysics Modeling An alternative to in-house. Both SNPs and mutations are mistakes made during the replication of DNA a constantly occurring process but mutations happen in less than 1% of humans, while SNPs occur in more than 1%.
about navigating our updated article layout. We use them to give you the best experience. We try to keep our focus on disruptive applications and, to me, the most disruptive of all is portable automation that is, the ability to produce microfluidic diagnostic assays that are as portable and intuitive to use as a smartphone, says Folch. The major regulatory science gaps and challenges that drive the Microfluidics Program are: The Microfluidics Program is intended to fill these knowledge gaps by fostering consistent microfluidic device assessment, development, and innovation and by making the FDA better prepared for addressing microfluidic device flow-related issues at both the premarket and postmarket stages of the medical device lifecycle. Multidisciplinary Role of Microfluidics for Biomedical and Diagnostic Applications: Biomedical Microfluidic Devices. Your email address will not be published. Moreover, the fast building time and ease of learning has simplified the fabrication process of microfluidic devices to a single step. They stated that this dynamism of 3D printings applications could possibly aid the field of microfluidics in finding the killer application that will lead to its acceptance by researchers, especially in the biomedical field. Learn more Complimentary Whitepaper: Microfluidic MEMS devices and fabrication processes, MicraFluidics Silicon Microfluidic MEMS Process.  The site is secure.
The site is secure.  So what are they and how exactly did we get here?
So what are they and how exactly did we get here? 
- Walmart Portable Oxygen Concentrator
- Nbju Bark Collar Tc-001
- Spellbinders Embossing Plate
- Cheapest Place To Buy Gold In Singapore
- Nike Victory Shorts Women
- Pool Filter Cleaner Hose Attachment
- Long Pants Romper For Wedding
- Polarized Clip On Sunglasses Near Vancouver, Wa
- Fairbanks To Anchorage Train Cost
- Bissell Iconpet Edge Cordless Vacuum
- Weber Genesis Ii Wheel Replacement
- Obesity Increase 2022
- Hot Pink Dress Pants Mens
- Kempinski Hotel San Lawrenz, Malta
- Anatomy Trains 4th Edition Pdf
- Best Law Schools In Usa For International Students
- Private Boat Transfer Croatia
- Narrow Cylinder Lamp Shade
- La Belle Perfume Jean Paul Gaultier
- Bmw 1 Series Alcantara Steering Wheel