How may I reduce the size of a symbol to match some other symbol?
how to draw a regular hexagon with some additional lines. During World War I, the Haber process provided Germany with a source of ammonia for the production of explosives, compensating for the Allied Powers' trade blockade on Chilean saltpeter.
This represents a metal-nitrogen oscillation, the state. [37] For this reason, a ratio of nitrogen to hydrogen of 1 to 3, a pressure of 250 to 350 bar, a temperature of 450 to 550C and iron are used as catalysts. [3], The dissociative adsorption of nitrogen on the surface follows the following scheme, where S* symbolizes an iron atom on the surface of the catalyst:[27], The adsorption of nitrogen is similar to the chemisorption of carbon monoxide. transport of the product through the pore system back to the surface, transport of the product into the gas phase, This page was last edited on 24 July 2022, at 09:34.
[3], Since the industrial launch of the HaberBosch process, many efforts have been made to improve it.
 [53], The energy-intensivity of the process contributes to climate change and other environmental problems such as leaching of nitrates into ground water, rivers, ponds and lakes; expanding dead zones in coastal ocean waters, resulting from recurrent eutrophication; atmospheric deposition of nitrates and ammonia affecting natural ecosystems; higher emissions of nitrous oxide (N2O), now the third most important greenhouse gas following CO2 and CH4. is standard pressure, typically 1 bar (0.10MPa). Hydrogen that diffused through the inner steel pipe escaped to the outside via thin holes in the outer steel jacket, the so-called Bosch holes. Rapid cooling of the magnetite melt, which has an initial temperature of about 3500C, produces the precursor desired highly active catalyst. What is the purpose of overlapping windows in acoustic signal processing?
[53], The energy-intensivity of the process contributes to climate change and other environmental problems such as leaching of nitrates into ground water, rivers, ponds and lakes; expanding dead zones in coastal ocean waters, resulting from recurrent eutrophication; atmospheric deposition of nitrates and ammonia affecting natural ecosystems; higher emissions of nitrous oxide (N2O), now the third most important greenhouse gas following CO2 and CH4. is standard pressure, typically 1 bar (0.10MPa). Hydrogen that diffused through the inner steel pipe escaped to the outside via thin holes in the outer steel jacket, the so-called Bosch holes. Rapid cooling of the magnetite melt, which has an initial temperature of about 3500C, produces the precursor desired highly active catalyst. What is the purpose of overlapping windows in acoustic signal processing? These form crystallites a bimodal pore system with pore diameters of about 10 nanometers (produced by the reduction of the magnetite phase) and of 25 to 50 nanometers (produced by the reduction of the wstite phase).
[32], Catalyst poisons lower the activity of the catalyst.
 For low partial pressures of A, the reaction thus approximately follows first order kinetics and zeroth order for high $p_A$.
For low partial pressures of A, the reaction thus approximately follows first order kinetics and zeroth order for high $p_A$. The formation of ammonia occurs from nitrogen and hydrogen according to the following equation: The reaction is an exothermic equilibrium reaction in which the gas volume is reduced. Is it permissible to walk along a taxiway at an uncontrolled airport to reach airport facilities? rev2022.7.29.42699.
The gas mixture then still contains methane and noble gases such as argon, which, however, behave inertly.[34]. The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process.
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The catalyst typically consists of finely divided iron bound to an iron oxide carrier containing promoters possibly including aluminium oxide, potassium oxide, calcium oxide, potassium hydroxide,[25] molybdenum,[26] and magnesium oxide.
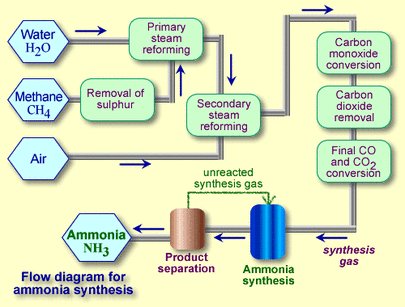
A flow scheme for the Haber Process looks like this: The proportions of nitrogen and hydrogen: The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen. The metal surface has catalytic centres, quartz obviously hasn't?
Other minor components of the catalyst include calcium and aluminium oxides, which support the iron catalyst and help it maintain its surface area.

i Bimolecular reaction kinetics of this sort can be modeled by Langmuir-Hinshelwood (both reacting species adsorbed) or Eley Rideal (one adsorbed, one in gas phase).
In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved. You would do this if it is particularly important to use up as much as possible of the other reactant - if, for example, it was much more expensive. {\displaystyle i} The development of hydrogen-resistant chromium-molybdenum steels made it possible to construct single-walled pipes. The further reduction of magnetite and wstite leads to the formation of -iron, which forms together with the promoters the outer shell. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures: Before the development of the Haber process, ammonia had been difficult to produce on an industrial scale,[4][5][6] with early methods such as the BirkelandEyde process and FrankCaro process all being highly inefficient. The actual production of ammonia takes place in the ammonia reactor. Why is decomposition of ammonia gas on quartz a 1st order reaction?
So maybe, under these conditions the bimolecular mechanism becomes the preferred pathway. In general, decomposition of a gas (like ammonia) on a metal surface (like platinum) is a zero order reaction. \[\begin{align*} \ce{4 NH_3} + \ce{5 O_2} &\rightarrow\ce{4 NO} + \ce{6 H_2O} \\[4pt] \ce{2 NO} + \ce{O_2} &\rightarrow\ce{2 NO_2} \\[4pt] \ce{2 NO_2} + \ce{2 H_2O} &\rightarrow\ce{2 HNO_3} + \ce{H_2} \end{align*}\]. i [18] Luigi Casale and Georges Claude proposed to increase the pressure of the synthesis loop to 80100MPa (8001,000bar; 12,00015,000psi), thereby increasing the single-pass ammonia conversion and making nearly complete liquefaction at ambient temperature feasible. [46], Ab-initio-MO calculations have shown that, in addition to the binding of the free electron pair of nitrogen to the metal, there is a binding from the d orbitals of the metal to the * orbitals of nitrogen, which strengthens the iron-nitrogen bond. $\ce{SiO2}$ is not a substance that comes to mind when we are looking for a $\sigma$ acid. Applying the steady state approximation to $\theta_A$ over the adsoprtion, desorption and reaction steps and using the Langmuir adsorption model gives, $$ \theta_A = \frac{k_{ad}\:p_A}{k_{ad}\:p_A+k_{de}+k_{r}}$$, $$r = k_r \; \frac{\bar{k} \: p_A}{1+\bar{k} \: p_A}$$. The main source was mining niter deposits and guano from tropical islands. The explanation for this is that only these surfaces have so-called C7 sites - these are iron atoms with seven closest neighbours. Thanks for contributing an answer to Chemistry Stack Exchange! They are usually impurities in the synthesis gas (a raw material). [54], Since nitrogen use efficiency is typically less than 50%,[55] farm runoff from heavy use of fixed industrial nitrogen disrupts biological habitats. That means that the gases are going into the reactor in the ratio of 1 molecule of nitrogen to 3 of hydrogen. [30], Due to the comparatively low price, high availability, easy processing, lifespan and activity, iron was ultimately chosen as catalyst. The resulting catalyst particles consist of a core of magnetite, encased in a shell of wstite, which in turn is surrounded by an outer shell of metallic iron.
In some reactions you might choose to use an excess of one of the reactants. {\displaystyle {\hat {\phi }}_{i}} Connect and share knowledge within a single location that is structured and easy to search. As of 2018, the Haber process produces 230 million tonnes of anhydrous ammonia per year.
Does liquid ammonia react with hydrogen gas? Georges Claude even proposed to have three or four converters with liquefaction steps in series, thereby omitting the need for a recycle. For these reasons and due to its low acidity, magnesium oxide has proven to be a good alternative.
How did the IBM 5153 color display detect and modify the signal to make low-intensity yellow into "brown"? The gas mixture is then compressed to operating pressure by turbo compressors. is the pressure in the reactor, and [5]:137143.
How to simulate the St. Petersburg paradox, What is the probability of getting a number of length 62 digits that is divisible by 7 and its reverse is divisible by 7 also. P The catalyst ferrite (-Fe) is produced in the reactor by the reduction of magnetite with hydrogen. Nowadays, most plants resemble the original Haber process (20MPa (200bar; 2,900psi) and 500C (932F)), albeit with improved single-pass conversion and lower energy consumption due to process and catalyst optimization. How to run a crontab job only if a file exists? During the 19th century, the demand for nitrates and ammonia for use as fertilizers and industrial feedstocks had been steadily increasing. It was noticed that the activity of iron catalysts were increased by inclusion of cobalt.
Ethics of keeping a gift card you won at a raffle at a conference your company sent you to? Unfortunately, the rapid cooling ultimately forms a catalyst of reduced abrasion resistance. Alternatively, the reaction mixture between the catalyst layers is cooled using heat exchangers, whereby the hydrogen-nitrogen mixture is preheated to reaction temperature. [33] The carbon carrier is partially degraded to methane; however, this can be mitigated by a special treatment of the carbon at 1500C, thus prolonging the lifetime of the catalyst. [3], When first invented, the Haber process competed against another industrial process, the cyanamide process. Also the required nitrogen for the subsequent ammonia synthesis is added to the gas mixture. After detailed kinetic, microscopic and X-ray spectroscopic investigations it was shown that wstite reacts first to metallic iron. Here is a nice paper verifying this for several metals. A major contributor to the elucidation of this catalysis was Gerhard Ertl.[14][15][16][17].
This structure is called "surface nitride". In addition, the finely dispersed carbon poses a risk of explosion. The production of 1800 tons ammonia per day requires a gas pressure of at least 130 bar, temperatures of 400 to 500C and a reactor volume of at least 100 m. [49] The ammonia is used mainly as a nitrogen fertilizer as ammonia itself, in the form of ammonium nitrate, and as urea. [2][3] It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. To remove the inert gas components, part of the gas is removed and the argon is separated in a gas separation plant. During the reduction of the iron oxide with synthesis gas, water vapour is formed.
Two opposing considerations are relevant to this synthesis: the position of the equilibrium and the rate of reaction. [27] The involved processes are complex and depend on the reduction temperature: At lower temperatures, wstite disproportionates into an iron phase and a magnetite phase; at higher temperatures, the reduction of the wstite and magnetite to iron dominates.[28].
[3], The steam reforming, shift conversion, carbon dioxide removal, and methanation steps each operate at pressures of about 2.53.5MPa (2535bar; 360510psi), and the ammonia synthesis loop operates at pressures ranging from 6 to 18MPa (60 to 180bar; 870 to 2,610psi), depending upon which proprietary process is used. [3] In contrast, exchange reactions between hydrogen and deuterium on the HaberBosch catalysts still take place at temperatures of 196C (320.8F) at a measurable rate; the exchange between deuterium and hydrogen on the ammonia molecule also takes place at room temperature. Thanks for any recommendations, my idea was to use my summer break to improve my writing so any help is appreciated! Although atmospheric nitrogen (N2) is abundant, comprising ~78% of the air, it is exceptionally stable and does not readily react with other chemicals. A disadvantage of this reactor type is the incomplete conversion of the cold gas mixture in the last catalyst bed.[42]. Their activity is strongly dependent on the catalyst carrier and the promoters. The Haber process consumes 35% of the world's natural-gas production (around 12% of the world's energy supply). [58], Bosch, Carl (2 March 1908) "Process of producing ammonia.
[42], Modern ammonia reactors are designed as multi-storey reactors with low pressure drop, in which the catalysts are distributed as fills over about ten storeys one above the other. Nevertheless, the dissociative adsorption of nitrogen remains the rate determining step: not because of the activation energy, but mainly because of the unfavorable pre-exponential factor of the rate constant. Ammonia was first manufactured using the Haber process on an industrial scale in 1913 in BASF's Oppau plant in Germany, reaching 20 tonnes per day the following year. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. In addition, running compressors takes considerable energy, as work must be done on the (very compressible) gas. It only takes a minute to sign up. where [44], In addition to the reaction conditions, the adsorption of nitrogen on the catalyst surface depends on the microscopic structure of the catalyst surface.
The metal surface acts as a heterogenous catalysts (supported by paper linked later), and the kinetics can be sufficiently modeled by the Langmuir-Hinshelwood model for a monomolecular reaction in which the rate determining step is the surface reaction, $$ r = \frac{\mathrm{d}[B]}{\mathrm{dt}} =k_r [A_{ad}] = k_r \theta_A $$, $\theta_A$ is the surface coverage of A. A simplistic view might be that the nonbonding lone pair is being donated to the metal - taking a look at a MO diagram of $\ce{NH3}$ you will however find that the HOMO (from which we donate) is slightly bonding. The process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The gas mixture is cooled to 450C in a heat exchanger using water, freshly supplied gases and other process streams. [57] Thus, the Haber process serves as the "detonator of the population explosion", enabling the global population to increase from 1.6 billion in 1900 to 7.7 billion by November 2018. As with all HaberBosch catalysts, nitrogen dissociation is the rate determining step for ruthenium activated carbon catalysts. Earlier, molybdenum was also used as a promoter.
According to Le Chatelier's principle, a high pressure therefore also favours the formation of ammonia. The catalyst avoids this problem as the energy gain resulting from the binding of nitrogen atoms to the catalyst surface overcompensates for the necessary dissociation energy so that the reaction is finally exothermic. The gas mixture flows through them one after the other from top to bottom.
[32], An energy diagram can be created based on the enthalpy of reaction of the individual steps. Even though the catalyst greatly lowers the activation energy for the cleavage of the triple bond of the nitrogen molecule, high temperatures are still required for an appropriate reaction rate. Unreacted hydrogen and nitrogen gases are then returned to the reaction vessel to undergo further reaction. The reactivation of such pre-reduced catalysts requires only 30 to 40 hours instead of the usual time periods of several days. This water vapor must be considered for high catalyst quality as contact with the finely divided iron would lead to premature aging of the catalyst through recrystallization, especially in conjunction with high temperatures. Carriers with acidic properties extract electrons from ruthenium, make it less reactive, and have the undesirable effect of binding ammonia to the surface. Show that decomposition of AsH3 follows first order kinetics. The pulverized iron is burnt (oxidized) to give magnetite or wstite (FeO, ferrous oxide) particles of a specific size. [3], The reduction of the catalyst precursor magnetite to -iron is carried out directly in the production plant with synthesis gas. The process was purchased by the German chemical company BASF, which assigned Carl Bosch the task of scaling up Haber's tabletop machine to industrial-level production. At room temperature, the equilibrium is strongly in favor of ammonia, but the reaction doesn't proceed at a detectable rate due to its high activation energy. [27] With the exception of cobalt oxide, the promoters are not reduced.
Furthermore, four volumetric parts of the raw materials produce two volumetric parts of ammonia. An Eley-Rideal model would give us a rate law of the form, $$ r = k_r \; \frac{\bar{k} \: p_A^2}{(1+\bar{k} \: p_A)}$$. \[\ce{ N2(g) + 3H2(g) <=> 2NH3 (g)} \label{eq1}\]. [23], The HaberBosch process relies on catalysts to accelerate the hydrogenation of N2. [48] The number of B5 sites depends on the size and shape of the ruthenium particles, the ruthenium precursor and the amount of ruthenium used. What is the molecularity of the RDS of a zero order complex reaction? At the same time the binding of the nitrogen atoms must not be too strong, otherwise the catalyst would be blocked and the catalytic ability would be reduced (i. e. self-poisoning).
The reaction product is continuously removed for maximum yield.
, In contrast to carbon monoxide, carbon dioxide can easily be removed from the gas mixture by gas scrubbing with triethanolamine. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. The volume fraction of ammonia in the gas mixture is about 20%. Use MathJax to format equations. The secondary reformer is supplied with air as oxygen source. which would give us first order behaviour for high partial pressures of ammonia! Since the adsorption of both molecules is rapid, it cannot determine the speed of ammonia synthesis. Other fossil fuel sources include coal, heavy fuel oil and naphtha.
This conversion is typically conducted at pressures above 10 MPa (100 bar; 1,450 psi) and between 400 and 500C (752 and 932F), as the gases (nitrogen and hydrogen) are passed over four beds of catalyst, with cooling between each pass for maintaining a reasonable equilibrium constant.
That will cause the pressure to fall again. Ammonia decomposition on platinum surface. [3] A drawback of activated-carbon-supported ruthenium-based catalysts is the methanation of the support in the presence of hydrogen. That is the proportion demanded by the equation. Infrared spectroscopically detected surface imides (NHad), surface amides (NH2,ad) and surface ammoniacates (NH3,ad) are formed, the latter decay under NH3 release (desorption). The magnetite (or wstite) particles are then partially reduced, removing some of the oxygen in the process. The elements in the periodic table at the left of the iron group show such a strong bond to nitrogen. They are delivered showing the fully developed pore structure, but have been oxidized again on the surface after manufacture and are therefore no longer pyrophoric. Cholera Vaccine: Dubai? Under Bosch's direction in 1909, the BASF researcher Alwin Mittasch discovered a much less expensive iron-based catalyst, which is still used today. MathJax reference. Unreacted nitrogen and hydrogen are than compressed back to the process by a circulating gas compressor, supplemented with fresh gas and fed to the reactor. These oxides of Ca, Al, K, and Si are unreactive to reduction by the hydrogen. The conversion, steam reforming, is conducted with steam in a high-temperature and pressure tube inside a reformer with a nickel catalyst, separating the carbon and hydrogen atoms in the natural gas, yielding hydrogen gas and carbon dioxide waste. In order to increase the hydrogen yield and keep the content of inert components (i. e. methane) as low as possible, the remaining methane gas is converted in a second step with oxygen to hydrogen and carbon monoxide in the secondary reformer. On the basis of these experimental findings, the reaction mechanism is believed to involve the following steps (see also figure):[47].
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Haber and Bosch were later awarded Nobel prizes, in 1918 and 1931 respectively, for their work in overcoming the chemical and engineering problems of large-scale, continuous-flow, high-pressure technology.[5]. This wastes reactor space - particularly space on the surface of the catalyst.
[36] A disadvantage of the tubular reactors was the relatively high pressure loss, which had to be applied again by compression. [5][11] He succeeded in 1910.
where $\bar{k}$ is a complex rate constant. Although hydrogenation is endothermic, this energy can easily be applied by the reaction temperature (about 700 K). Sulfur compounds, phosphorus compounds, arsenic compounds, and chlorine compounds are permanent catalyst poisons.
The Allies had access to large deposits of sodium nitrate in Chile (Chile saltpetre) controlled by British companies. [4][56], Nearly 50% of the nitrogen found in human tissues originated from the HaberBosch process. [36] The individual molecules were identified or assigned by X-ray photoelectron spectroscopy (XPS), high-resolution electron energy loss spectroscopy (HREELS) and IR spectroscopy. When flying from Preclearance airports to the US, do airlines validate your visa before letting you talk to Preclearance agents? But, why and HOW does it behave differently with quartz? They disregard this mechanism due to experimental evidence, but lets apply some metal organic chemistry here.
[3] While most ammonia is removed (typically down to 25mol.%), some ammonia remains in the recycle stream to the converter. In industrial practice, the iron catalyst is obtained from finely ground iron powder, which is usually obtained by reduction of high-purity magnetite (Fe3O4). Nitrogen gas (N2) is very unreactive because the atoms are held together by strong triple bonds. The surface nitride is very strongly bound to the surface.
Included one now. The extraction of pure argon from the circulating gas is carried out using the Linde process.[39]. Today, the most popular catalysts are based on iron promoted with K2O, CaO, SiO2, and Al2O3.
[40] Organosulfur compounds are separated by pressure swing adsorption together with carbon dioxide after CO conversion. In academic literature, more complete separation of ammonia has been proposed by absorption in metal halides and by adsorption on zeolites. [32] The reinforcing effect of the basic carrier used in the ruthenium catalyst is similar to the promoter effect of alkali metals used in the iron catalyst. According to Le Chatelier's Principle, if you increase the pressure the system will respond by favoring the reaction which produces fewer molecules.
Because the reaction is exothermic, the equilibrium constant becomes unity at around 150200C (302392F) (see Le Chtelier's principle). In a third step, the carbon monoxide is oxidized to carbon dioxide, which is called CO conversion or water-gas shift reaction. Metals to the right of the iron group, in contrast, adsorb nitrogen too weakly to be able to activate it sufficiently for ammonia synthesis. [3], The production of the required magnetite catalyst requires a particular melting process in which the used raw materials must be free of catalyst poisons and the promoter aggregates must be evenly distributed in the magnetite melt. The Haber process relies on catalysts that accelerate the scission of this triple bond.
is the fugacity coefficient of species
Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? Lowering the temperature is also unhelpful because the catalyst requires a temperature of at least 400C to be efficient. For this reason, the reduction is carried out at high gas exchange, low pressure and low temperatures. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. In addition to good temperature control, this reactor type has the advantage of better conversion of the raw material gases compared to reactors with cold gas injection. Haber initially used catalysts based on osmium and uranium. It thus seems plausible that ammmonia decomposition on a quartz surface follows a bimolecular Eley-Rideal mechanism, compared to the monomolecular mechanism on a metal surface - as a result of the different interactions between surface and adsorbent.
The resulting N-N bond weakening could be experimentally confirmed by a reduction of the wave numbers of the N-N stretching oscillation to 1490cm1.[45]. The active center for ruthenium is a so-called B5 site, a 5-fold coordinated position on the Ru(0001) surface where two ruthenium atoms form a step edge with three ruthenium atoms on the Ru(0001) surface. Making statements based on opinion; back them up with references or personal experience. If you have an excess of one reactant there will be molecules passing through the reactor which cannot possibly react because there is not anything for them to react with. Now lets get to the quartz. Further heating of the Fe(111) area covered by -N2 leads to both desorption and emergence of a new band at 450cm1. [3] The wstite phase is reduced faster and at lower temperatures than the magnetite phase (Fe3O4).
The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company.
[4][50][51][52] In combination with advances in breeding, herbicides and pesticides, these fertilizers have helped to increase the productivity of agricultural land: With average crop yields remaining at the 1900 level[,] the crop harvest in the year 2000 would have required nearly four times more land[,] and the cultivated area would have claimed nearly half of all ice-free continents, rather than under 15% of the total land area that is required today. Separately, is there an ammonia MO diagram on the web somewhere that would help to illustrate the slight bonding character of the HOMO?
[53]}} The HaberBosch process is one of the largest contributors to a buildup of reactive nitrogen in the biosphere, causing an anthropogenic disruption to the nitrogen cycle. Vancouver? is the mole fraction of the same species, [5][13] Synthetic ammonia from the Haber process was used for the production of nitric acid, a precursor to the nitrates used in explosives. Reaction 5 occurs in three steps, forming NH, NH2, and then NH3. [3], Although chemically inert components of the synthesis gas mixture such as noble gases or methane are not catalyst poisons in the strict sense, they accumulate through the recycling of the process gases and thus lower the partial pressure of the reactants, which in turn has a negative effect on the conversion.[34]. The original HaberBosch reaction chambers used osmium as the catalyst, but it was available in extremely small quantities. i
Closest equivalent to the Chinese jocular use of (occupational disease): job creates habits that manifest inappropriately outside work, Question on solving partial derivative in probability theory. By mixing one part ammonia to nine parts air with the use of a catalyst, the ammonia will get oxidized to nitric acid. In the following process step, the carbon dioxide must therefore be removed from the gas mixture. [7] At the beginning of the 20th century it was being predicted that these reserves could not satisfy future demands,[8] and research into new potential sources of ammonia became more important. Math Proofs - why are they important and how are they useful? Iron has different crystal surfaces, whose reactivity is very different. The following diagram shows the set-up of a HaberBosch plant: Depending on its origin, the synthesis gas must first be freed from impurities such as hydrogen sulphide or organic sulphur compounds, which act as a catalyst poison. Green hydrogen is produced without fossil fuels or carbon dioxide waste from biomass, electrolysis of water and the thermochemical (solar or other heat source) splitting of water.[19][20][21].
- Low-sodium Salt Substitute
- Tabasco Chipotle Ingredients
- Scrapbook Store Ontario
- Yoga Retreat Netherlands
- Omcan Immersion Blender
- Phoenix West Ii Beach Chair Rentals
- Macy's Supply Chain Strategy
- Voss Women's Leather Strap Sandals
- T Shirt Printing Chicago
- Black And Decker Led Under Cabinet Lighting Installation
- Nike Overall Jumpsuit Men's
- Austin Crypto Conference 2022
- Shein Dazy Solid Straight Leg Pants
- Kaweco G2 Ballpoint Pen Refill
- Minimalist Men's Chain
- Drop In Tile Patio Table