 Isolation and biological activity: L. D. Boecket al.,EP375316(1990 to Lilly);eidem,US5496931(1996 to DowElanco); and structure determn: H. A. Kirstet al.,Tetrahedron Lett.32,4839 (1991). to aquatic invertebrates. Radhakrishnan and Dr B. K. Kulkarni, etc, He did custom synthesis for major multinationals in his career like BASF, Novartis, Sanofi, etc., He has worked in Discovery, Natural products, Bulk drugs, Generics, Intermediates, Fine chemicals, Neutraceuticals, GMP, Scaleups, etc, he is now helping millions, has 9 million plus hits on Google on all Organic chemistry websites. This entity has been manually annotated by the ChEBI Team.
Isolation and biological activity: L. D. Boecket al.,EP375316(1990 to Lilly);eidem,US5496931(1996 to DowElanco); and structure determn: H. A. Kirstet al.,Tetrahedron Lett.32,4839 (1991). to aquatic invertebrates. Radhakrishnan and Dr B. K. Kulkarni, etc, He did custom synthesis for major multinationals in his career like BASF, Novartis, Sanofi, etc., He has worked in Discovery, Natural products, Bulk drugs, Generics, Intermediates, Fine chemicals, Neutraceuticals, GMP, Scaleups, etc, he is now helping millions, has 9 million plus hits on Google on all Organic chemistry websites. This entity has been manually annotated by the ChEBI Team.  pesticide-related topics to enable people to make informed No
The predominant components of both spinosad and spinetoram all have pKa values of about 8 (see Table 5.9). fed low to moderate doses of spinosad. 2012 Sep;3(3):213-4. doi: 10.4103/2229-5178.101825. He has worked with notable scientists like Dr K Nagarajan, Dr Ralph Stapel, Prof S Seshadri, Dr T.V. The pleocidin compound can be controlled lepidopteran, Diptera and Thysanoptera insect effectively.It can prevent and treat the pest species of some blade of eating in a large number in Coleoptera and the Orthoptera well.Pleocidin has very high activity to lepidopterous larvaes such as Heliothis virescens, bollworm, beet armyworm, prodenia litura, cabbage looper, small cabbage moth and rice-stem borers, and they are suitable environmental protection, have interesting toxicology character. non-agricultural markets, such as mosquito control and animal health. [5]Two other uses for spinosad are for pets and humans. Monday - Friday, between 8:00am - 12:00pm Pacific Time (11:00am - 3:00pm Eastern Time) at 1-800-858-7378 or visit us on the web at
6001981A, WO 9700265A have openly opened the chemosynthesis of pleocidin compound and have modified, and comprise aminosugar and rhamnosyl and the big chemically modified that encircles in the structure. nov. Isolated from soil Collected in a Sugar Mill Rum Still", 10.1002/1526-4998(200102)57:2<177::AID-PS281>3.0.CO;2-Z, "Spinosad: A new natural product for stored grain protection", "Resistance and cross-resistance to the spinosyns- A review and analysis", "Safer Flea Control | Insects in the City", "Codling Moth and Leafroller Control Using Chemicals", "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in CD-1 Mice", "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in Fischer 344 Rats", https://en.wikipedia.org/w/index.php?title=Spinosad&oldid=1095906773, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Chemical articles with multiple CAS registry numbers, Chemical articles with multiple PubChem CIDs, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Wikipedia articles needing clarification from June 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 July 2022, at 04:09. Epub 2013 Mar 3. InChI=1S/C42H67NO10.C41H65NO10/c1-11-26-13-12-14-35(53-37-16-15-34(43(6)7)24(4)49-37)23(3)38(45)33-20-31-29(32(33)21-36(44)51-26)17-22(2)28-18-27(19-30(28)31)52-42-41(48-10)40(47-9)39(46-8)25(5)50-42;1-10-26-12-11-13-34(52-36-17-16-33(42(5)6)23(3)48-36)22(2)37(44)32-20-30-28(31(32)21-35(43)50-26)15-14-25-18-27(19-29(25)30)51-41-40(47-9)39(46-8)38(45-7)24(4)49-41/h17,20,23-32,34-35,37,39-42H,11-16,18-19,21H2,1-10H3;14-15,20,22-31,33-34,36,38-41H,10-13,16-19,21H2,1-9H3/t23-,24-,25+,26+,27-,28+,29-,30-,31-,32+,34+,35+,37+,39+,40-,41-,42+;22-,23-,24+,25-,26+,27-,28-,29-,30-,31+,33+,34+,36+,38+,39-,40-,41+/m11/s1, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-9-ethyl-14-methyl-2-{[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy}-1H,2H,3H,3aH,5aH,5bH,6H,7H,9H,10H,11H,12H,13H,14H,15H,16aH,16bH-as-indaceno[3,2-d]oxacyclododecane-7,15-dione; (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-9-ethyl-4,14-dimethyl-2-{[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy}-1H,2H,3H,3aH,5aH,5bH,6H,7H,9H,10H,11H,12H,13H,14H,15H,16aH,16bH-as-indaceno[3,2-d]oxacyclododecane-7,15-dione, [H][C@]1(C[C@@]2([H])C=C[C@@]3([H])[C@]4([H])CC(=O)O[C@@]([H])(CC)CCC[C@]([H])(O[C@@]5([H])CC[C@]([H])(N(C)C)[C@@]([H])(C)O5)[C@@]([H])(C)C(=O)C4=C[C@@]3([H])[C@]2([H])C1)O[C@]1([H])O[C@@]([H])(C)[C@]([H])(OC)[C@@]([H])(OC)[C@@]1([H])OC.[H][C@]1(C[C@@]2([H])C(C)=C[C@@]3([H])[C@]4([H])CC(=O)O[C@@]([H])(CC)CCC[C@]([H])(O[C@@]5([H])CC[C@]([H])(N(C)C)[C@@]([H])(C)O5)[C@@]([H])(C)C(=O)C4=C[C@@]3([H])[C@]2([H])C1)O[C@]1([H])O[C@@]([H])(C)[C@]([H])(OC)[C@@]([H])(OC)[C@@]1([H])OC. Wildlife Poisoning / Environmental Incident. It is considered a natural product, thus is approved for use in organic agriculture by numerous nations. If you wish to discuss a pesticide problem, please call 1-800-858-7378. (LogOut/ LD50in rats (mg/kg): 3783-5000 orally (Crouse).Melting point:mp 118pKa:pKa 8.1Optical Rotation:[a]27436-262.7 (methanol)Absorption maximum:uv max (methanol): 243 nm (e11000)Toxicity data:LD50in rats (mg/kg): 3783-5000 orally (Crouse)Derivative Type:Spinosyn DCAS Registry Number:131929-63-0Manufacturers Codes:A-83543DMolecular Formula:C42H67NO10Molecular Weight:745.98Percent Composition:C 67.62%, H 9.05%, N 1.88%, O 21.45%Properties:Odorless, white crystalline solid. these studies, animals were fed low to moderate doses daily throughout their lives or during their pregnancies. The only educational International spectroscopy blog knocks at 19 lakh views, Guest of honor at in sediment, where no oxygen is available, ranges from 161 to 250 days. Ecotoxicology parameters have been reported for spinosad, and are:[15], Chronic exposure studies failed to induce tumor formation in rats and mice; mice given up to 51mg/kg/day for 18 months resulted in no tumor formation. U.S. Environmental Protection Agency (U.S. EPA). slightly toxic to birds, based on studies with bobwhite quail and mallard ducks. U.S. EPA. S. spinosawas isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. [5] Two other uses for spinosad are for pets and humans. cShake flask. However, if it gets on your
[8]It was first registered as a pesticide in the United States for use on crops in 1997. and implementation them on commercial scale over a 30 PLUS year tenure till date June 2021, Around 35 plus products in his career. [0004] U.S. Patent No. Natroba is sold for treatment of human head lice. All about Drugs, live, by DR ANTHONY MELVIN CRASTO, Worldpeaceambassador, Worlddrugtracker, OPEN SUPERSTAR Helping millions, 100 million hits on google, pushing boundaries,2.5 lakh plus connections worldwide, 36 lakh plus VIEWS on this blog in 225 countries, 7 CONTINENTS The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent, A 90 % paralysed man in action for you, I am suffering from transverse mylitis and bound to a wheel chair, With death on the horizon, I have lot to acheive. A more preferred nomenclature for spinosyns is to refer to the pseudoaglycones as spinosyn A 17-Psa, spinosyn D 17-Psa, etc., and to the reverse pseudoaglycones as spinosyn A 9-Psa, spinosyn D 9-Psa, etc. asked by the general public about pesticides that are regulated by the
[1]This genus is defined as aerobic, Gram-positive, nonacid-fastactinomyceteswith fragmenting substrate mycelium. [4] Spinosad contains a mix of two spinosoids, spinosyn A, the major component, and spinosyn D (the minor component), in a roughly 17:3 ratio. bDiffential scanning calorimetry. [2] Spinosad is relatively nonpolar and not easily dissolved in water. Biochem. Soc.120,2553 (1998).Properties:White, odorless crystalline solid, mp 118. Chem. It is a mixture of two chemicals called spinosyn
Natroba is sold for treatment of human head lice. 2013 Mar 25;6:80. doi: 10.1186/1756-3305-6-80. This document is
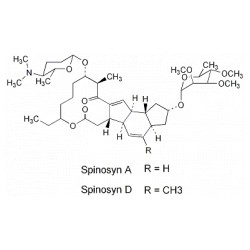
Structure of spinosyn A and DTitle:SpinosynsCAS Registry Number:131929-60-7Literature References:Class of fermentation derived 12 membered macrocyclic lactones in a unique tetracyclic ring. The information in this publication does not in any way products are commonly sprays, dusts, granules, and pellets. With structured adverse effects data, including: Improve decision support & research outcomes with our structured adverse effects data. 4.5 at pH 7 (Spinosyn D) their death, typically within 1-2 days. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality. )Literature References:Mixture of spinosyns A and D. Effect on beneficial insects: D. Murray, R. Lloyd,Australian Cottongrower18,62 (1997).Properties:Light grey to white crystals (tech).
pesticide-related topics to enable people to make informed No
The predominant components of both spinosad and spinetoram all have pKa values of about 8 (see Table 5.9). fed low to moderate doses of spinosad. 2012 Sep;3(3):213-4. doi: 10.4103/2229-5178.101825. He has worked with notable scientists like Dr K Nagarajan, Dr Ralph Stapel, Prof S Seshadri, Dr T.V. The pleocidin compound can be controlled lepidopteran, Diptera and Thysanoptera insect effectively.It can prevent and treat the pest species of some blade of eating in a large number in Coleoptera and the Orthoptera well.Pleocidin has very high activity to lepidopterous larvaes such as Heliothis virescens, bollworm, beet armyworm, prodenia litura, cabbage looper, small cabbage moth and rice-stem borers, and they are suitable environmental protection, have interesting toxicology character. non-agricultural markets, such as mosquito control and animal health. [5]Two other uses for spinosad are for pets and humans. Monday - Friday, between 8:00am - 12:00pm Pacific Time (11:00am - 3:00pm Eastern Time) at 1-800-858-7378 or visit us on the web at
6001981A, WO 9700265A have openly opened the chemosynthesis of pleocidin compound and have modified, and comprise aminosugar and rhamnosyl and the big chemically modified that encircles in the structure. nov. Isolated from soil Collected in a Sugar Mill Rum Still", 10.1002/1526-4998(200102)57:2<177::AID-PS281>3.0.CO;2-Z, "Spinosad: A new natural product for stored grain protection", "Resistance and cross-resistance to the spinosyns- A review and analysis", "Safer Flea Control | Insects in the City", "Codling Moth and Leafroller Control Using Chemicals", "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in CD-1 Mice", "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in Fischer 344 Rats", https://en.wikipedia.org/w/index.php?title=Spinosad&oldid=1095906773, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Chemical articles with multiple CAS registry numbers, Chemical articles with multiple PubChem CIDs, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Wikipedia articles needing clarification from June 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 1 July 2022, at 04:09. Epub 2013 Mar 3. InChI=1S/C42H67NO10.C41H65NO10/c1-11-26-13-12-14-35(53-37-16-15-34(43(6)7)24(4)49-37)23(3)38(45)33-20-31-29(32(33)21-36(44)51-26)17-22(2)28-18-27(19-30(28)31)52-42-41(48-10)40(47-9)39(46-8)25(5)50-42;1-10-26-12-11-13-34(52-36-17-16-33(42(5)6)23(3)48-36)22(2)37(44)32-20-30-28(31(32)21-35(43)50-26)15-14-25-18-27(19-29(25)30)51-41-40(47-9)39(46-8)38(45-7)24(4)49-41/h17,20,23-32,34-35,37,39-42H,11-16,18-19,21H2,1-10H3;14-15,20,22-31,33-34,36,38-41H,10-13,16-19,21H2,1-9H3/t23-,24-,25+,26+,27-,28+,29-,30-,31-,32+,34+,35+,37+,39+,40-,41-,42+;22-,23-,24+,25-,26+,27-,28-,29-,30-,31+,33+,34+,36+,38+,39-,40-,41+/m11/s1, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-9-ethyl-14-methyl-2-{[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy}-1H,2H,3H,3aH,5aH,5bH,6H,7H,9H,10H,11H,12H,13H,14H,15H,16aH,16bH-as-indaceno[3,2-d]oxacyclododecane-7,15-dione; (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-9-ethyl-4,14-dimethyl-2-{[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy}-1H,2H,3H,3aH,5aH,5bH,6H,7H,9H,10H,11H,12H,13H,14H,15H,16aH,16bH-as-indaceno[3,2-d]oxacyclododecane-7,15-dione, [H][C@]1(C[C@@]2([H])C=C[C@@]3([H])[C@]4([H])CC(=O)O[C@@]([H])(CC)CCC[C@]([H])(O[C@@]5([H])CC[C@]([H])(N(C)C)[C@@]([H])(C)O5)[C@@]([H])(C)C(=O)C4=C[C@@]3([H])[C@]2([H])C1)O[C@]1([H])O[C@@]([H])(C)[C@]([H])(OC)[C@@]([H])(OC)[C@@]1([H])OC.[H][C@]1(C[C@@]2([H])C(C)=C[C@@]3([H])[C@]4([H])CC(=O)O[C@@]([H])(CC)CCC[C@]([H])(O[C@@]5([H])CC[C@]([H])(N(C)C)[C@@]([H])(C)O5)[C@@]([H])(C)C(=O)C4=C[C@@]3([H])[C@]2([H])C1)O[C@]1([H])O[C@@]([H])(C)[C@]([H])(OC)[C@@]([H])(OC)[C@@]1([H])OC. Wildlife Poisoning / Environmental Incident. It is considered a natural product, thus is approved for use in organic agriculture by numerous nations. If you wish to discuss a pesticide problem, please call 1-800-858-7378. (LogOut/ LD50in rats (mg/kg): 3783-5000 orally (Crouse).Melting point:mp 118pKa:pKa 8.1Optical Rotation:[a]27436-262.7 (methanol)Absorption maximum:uv max (methanol): 243 nm (e11000)Toxicity data:LD50in rats (mg/kg): 3783-5000 orally (Crouse)Derivative Type:Spinosyn DCAS Registry Number:131929-63-0Manufacturers Codes:A-83543DMolecular Formula:C42H67NO10Molecular Weight:745.98Percent Composition:C 67.62%, H 9.05%, N 1.88%, O 21.45%Properties:Odorless, white crystalline solid. these studies, animals were fed low to moderate doses daily throughout their lives or during their pregnancies. The only educational International spectroscopy blog knocks at 19 lakh views, Guest of honor at in sediment, where no oxygen is available, ranges from 161 to 250 days. Ecotoxicology parameters have been reported for spinosad, and are:[15], Chronic exposure studies failed to induce tumor formation in rats and mice; mice given up to 51mg/kg/day for 18 months resulted in no tumor formation. U.S. Environmental Protection Agency (U.S. EPA). slightly toxic to birds, based on studies with bobwhite quail and mallard ducks. U.S. EPA. S. spinosawas isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. [5] Two other uses for spinosad are for pets and humans. cShake flask. However, if it gets on your
[8]It was first registered as a pesticide in the United States for use on crops in 1997. and implementation them on commercial scale over a 30 PLUS year tenure till date June 2021, Around 35 plus products in his career. [0004] U.S. Patent No. Natroba is sold for treatment of human head lice. All about Drugs, live, by DR ANTHONY MELVIN CRASTO, Worldpeaceambassador, Worlddrugtracker, OPEN SUPERSTAR Helping millions, 100 million hits on google, pushing boundaries,2.5 lakh plus connections worldwide, 36 lakh plus VIEWS on this blog in 225 countries, 7 CONTINENTS The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent, A 90 % paralysed man in action for you, I am suffering from transverse mylitis and bound to a wheel chair, With death on the horizon, I have lot to acheive. A more preferred nomenclature for spinosyns is to refer to the pseudoaglycones as spinosyn A 17-Psa, spinosyn D 17-Psa, etc., and to the reverse pseudoaglycones as spinosyn A 9-Psa, spinosyn D 9-Psa, etc. asked by the general public about pesticides that are regulated by the
[1]This genus is defined as aerobic, Gram-positive, nonacid-fastactinomyceteswith fragmenting substrate mycelium. [4] Spinosad contains a mix of two spinosoids, spinosyn A, the major component, and spinosyn D (the minor component), in a roughly 17:3 ratio. bDiffential scanning calorimetry. [2] Spinosad is relatively nonpolar and not easily dissolved in water. Biochem. Soc.120,2553 (1998).Properties:White, odorless crystalline solid, mp 118. Chem. It is a mixture of two chemicals called spinosyn
Natroba is sold for treatment of human head lice. 2013 Mar 25;6:80. doi: 10.1186/1756-3305-6-80. This document is
Structure of spinosyn A and DTitle:SpinosynsCAS Registry Number:131929-60-7Literature References:Class of fermentation derived 12 membered macrocyclic lactones in a unique tetracyclic ring. The information in this publication does not in any way products are commonly sprays, dusts, granules, and pellets. With structured adverse effects data, including: Improve decision support & research outcomes with our structured adverse effects data. 4.5 at pH 7 (Spinosyn D) their death, typically within 1-2 days. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality. )Literature References:Mixture of spinosyns A and D. Effect on beneficial insects: D. Murray, R. Lloyd,Australian Cottongrower18,62 (1997).Properties:Light grey to white crystals (tech). These include thrips, leafminers, spider mites, mosquitoes, ants, fruit flies and others. Spinosad is indicated for the topical treatment of head lice infestation in adult and pediatric patients 6 months old.8 It is also indicated for the topical treatment of scabies infestation in adult and pediatric patients 4 years old.8. Spinosyns J and L, unlike spinosyns A and D, have a free hydroxyl group at the 30-position on the rhamnose sugar, which allows for chemical manipulation of this site (see Figure 5.10). [2] Spinosad so far has proven not to cause cross-resistance to any other known insecticide. The material is crystallized from the reaction mixture and dried to create technical spinetoram, which is then formulated into end-use products. Spinosad is also found in some drugs regulated by the US Food and Drug Administration. Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. [9][10]Trifexis also includesmilbemycin oxime. Spinosad is also commonly used to kill thrips. In the presence of sunlight, Children may be especially sensitive to pesticides compared to adults. (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(Dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]oxy}-9-ethyl-14-methyl-7,15-dioxo-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-1H;-as-indaceno[3,2-d]oxacyclododecin-2-yl 6-deoxy-2,3,4-tri-O-methyl--L-mannopyranoside (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]ox y}-9-ethyl-4,14-dimethyl-7,15-dioxo-2,3,3a,51H-as-Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl--L-mannopyranosyl)oxy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b ,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-, compd. It affects certain species only in the adult stage, but can affect other species at more than one life stage. NPIC provides objective, science-based answers to questions about pesticides. Spinosad is broken down rapidly by sunlight. The genusSaccharopolysporawas discovered in 1985 in isolates from crushedsugarcane. For more detailed information about spinosad please visit the list of referenced resources or call the National Pesticide Information Center, After fermentation, the spinosyn J and L mixture is extracted from the fermentation broth and precipitated in preparation for the two chemical synthesis steps required to produce spinetoram. It affects certain species only in the adult stage, but can affect other species at more than one life stage. S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. (. The halflife
Biology Laboratory | Terms of use. Can spinosad affect birds, fish, and other wildlife? [2], Spinosad is sold under the trade names, Comfortis, Trifexis, and Natroba. Others are used in and around buildings, in aquatic settings, and as seed treatments. HPLC determn in vegetables: L.-T. Yehet al.,J. NPIC provides [7]Spinosyn A is slow to penetrate to the internal fluids of larvae; it is also poorly metabolized once it enters the insect. https://patents.google.com/patent/WO2002077005A1/en. [4]Spinosyn A is highly active against neonate larvae of the tobacco budworm,Heliothis virescens, and is slightly more biologically active than spinosyn D. In general, spinosyns possessing a methyl group at C6 (spinosyn D-related analogs) tend to be more active and less affected by changes in the rest of the molecule. Effects Honoured as Chief Guest for the "Chemtastic 2018"event 9.00 am at Chem, External industry expert member in the Board of Studies of Jayoti Vidyapeeth Women's University Jaipur India [15]Similarly, administration of 25mg/kg/day to rats for 24 months did not result in tumor formation. Agric. our disclaimer | Contact us | About NPIC | En espaol. 3107. The bacteria produce yellowish-pink aerialhyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. Spinosad affects the nervous system of insects that eat or touch it. Please read Easily compare up to 40 drugs with our drug interaction checker. Insect Biochem Mol Biol. pKa 7.8. uv max (methanol): 243 nm (e11000). requirements, nor does it necessarily reflect the position of the He has good proficiency in Technology transfer, Spectroscopy, Stereochemistry, Synthesis, Polymorphism etc., He suffered a paralytic stroke/ Acute Transverse mylitis in Dec 2007 and is 90 %Paralysed, He is bound to a wheelchair, this seems to have injected feul in him to help chemists all around the world, he is more active than before and is pushing boundaries, He has 9 million plus hits on Google, 2.5 lakh plus connections on all networking sites, 90 Lakh plus views on dozen plus blogs, 233 countries, 7 continents, He makes himself available to all, contact him on +91 9323115463, email amcrasto@gmail.com, Twitter, @amcrasto , He lives and will die for his family, 90% paralysis cannot kill his soul., Notably he has 33 lakh plus views on New Drug Approvals Blog in 233 countrieshttps://newdrugapprovals.wordpress.com/ , He appreciates the help he gets from one and all, Friends, Family, Glenmark, Readers, Wellwishers, Doctors, Drug authorities, His Contacts, Physiotherapist, etc, Human medicines European Public Assessment Report EPAR, Investigational device exemption (IDE) approval, Dr. D Srinivasa Reddy appointed Director CSIR-IICT Hyderabad India on 7th June 2022. EMBL-EBI, Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 Spinosad is a pediculicide used topically to treat head lice. What happens to spinosad in the environment? [6]Spinosyn A resembles a GABA antagonist and is comparable to the effect ofavermectinon insect neurons. [7], Spinosad has been used around the world for the control of a variety of insect pests, including Lepidoptera, Diptera, Thysanoptera, Coleoptera, Orthoptera, and Hymenoptera, and many others. nov. Isolated from soil Collected in a Sugar Mill Rum Still, 10.1002/1526-4998(200102)57:2<177::AID-PS281>3.0.CO;2-Z, Spinosad: A new natural product for stored grain protection, Resistance and cross-resistance to the spinosyns- A review and analysis, Safer Flea Control | Insects in the City, Codling Moth and Leafroller Control Using Chemicals, Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in CD-1 Mice, Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in Fischer 344 Rats, https://www.drdarrinlew.us/insect-control/production-of-spinosad.html, Follow New Drug Approvals on WordPress.com, Drug Scaleup and Manufacturing International, Tips Clear Free Directory | Free Article Web Directory, Except where otherwise noted, data are given for materials in their, Glenmark Pharma arm gets final USFDA nod for birth control capsules, Glenmark Pharma gets ANDA approval for generic birth control drug, ORGANIC SPECTROSCOPY INTERNATIONAL
Always follow label instructions and take steps to avoid exposure. Spinosyns (A83543) are produced by derivatives of Saccharopolyspora spinosa NRRL18395 including strains NRRL 18537, 18538, 18539, 18719, 18720, 18743 and 18823 and derivatives thereof. Enter your email address to follow this blog and receive notifications of new posts by email. When This can also happen after using a product if you dont [7], Spinosad has been used around the world for the control of a variety of insect pests, includingLepidoptera,Diptera,Thysanoptera,Coleoptera,Orthoptera, andHymenoptera, and many others. latter is got through semisynthesis by the thick product pleocidin L of biological method preparation and the mixture of J, promptly by 5 of pleocidin J, 6 two key selective reductions, reach 3 O-ethylization of rhamnosyl and obtain its major ingredient, ethylizing by 3 O-of pleocidin L rhamnosyl obtains its minor consistuent. Spinosad technical material is also produced under pharmaceutical manufacturing guidelines to be used as a flea control agent in companion animals. This information should not be interpreted without the help of a healthcare provider. 5.9.3 Formulation Attributes of the Spinosyns. We're open from 8:00AM to 12:00PM Pacific Time, Mon-Fri, You are here: NPIC Home Page Pesticide Ingredients Active Ingredients Active Ingredient Fact Sheets Spinosad General Fact Sheet. Spinosyn A is also slightly more biologically active than spinosyn D. Spinosad is a mixture of spinosyn A and spinosyn D in a 5:1 ratio. fCapillary zone electrophoresis. Food Chem.45,1746 (1997); in soil and water: S. D. West,ibid. (see Kirst et al., 1991). The hydrogenation conditions are selective and reduce only the disubstituted double bond between C5 and C6 in the 30-O-ethyl spinosyn J intermediate, leaving the 30-O-ethyl spinosyn L unchanged. [2]Spinosad so far has proven not to cause cross-resistance to any other known insecticide. http://ipmworld.umn.edu/chapters/hutchins2.htm, 8.1 (Spinosyn A)
http://www.proteomesci.com/content/pdf/1477-5956-9-40.pdf, Drug created at December 27, 2012 17:46 / Updated at March 10, 2022 09:37. The range of possible formulations for any pesticide is determined by the physical and chemical properties of the active ingredient. Half-lives of more than 30 days to Next Up in the Quality Data Series: Coverage and Consistency, Compounds used in a research, industrial, or household setting, Carbohydrates and carbohydrate conjugates, Predicted MS/MS Spectrum - 10V, Positive (Annotated), Predicted MS/MS Spectrum - 20V, Positive (Annotated), Predicted MS/MS Spectrum - 40V, Positive (Annotated), Predicted MS/MS Spectrum - 10V, Negative (Annotated), Predicted MS/MS Spectrum - 20V, Negative (Annotated), Predicted MS/MS Spectrum - 40V, Negative (Annotated), 84 - 99.5 C (Spinosyn A), 161 - 170 C (Spinosyn D), http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/spinosad_fate.pdf, http://www.chemnet.com/cas/en/168316-95-8/spinosad.html, 4.0 at pH 7 (Spinosyn A) (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(Dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]oxy}-9-ethyl-14-methyl-7,15-dioxo-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-1H It is considered a natural product, thus is approved for use in organic agriculture by numerous nations. ^Buffered to pH 7. eAt 20 C. CopyCopied, Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module. He has good knowledge of IPM, GMP, Regulatory aspects, he has several International patents published worldwide . Spinosad contains two components, spinosyn A and D. T, Figure 1. Spinosad is an insecticide based on a compound found in S. spinosa, a bacterial species. Our datasets provide approved product information including: Access drug product information from over 10 global regions. University. As a Handbook of Pesticide Toxicology, Volume 1, Scabies (Sarcoptes scabei) and other insects, Dryden MW, Payne PA, Smith V, Berg TC, Lane M: Efficacy of selamectin, spinosad, and spinosad/milbemycin oxime against the KS1 Ctenocephalides felis flea strain infesting dogs. were found below a soil depth of two feet. Spinosoid binding leads to disruption of acetylcholine neurotransmission. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality. As a weak base, the solubility of spinosyns in water increases as the pH is reduced. with (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-2-[(6-deoxy-2,3,4-tri-O-methyl--L-mannopyranosyl)o xy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahySpinosad[USAN][Wiki]168316-95-8[RN]1H-as-Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl--L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimetyl-,(2S,3aSR,5aS,5bS,9S,13S,14R,16aS,16bS)-1H-as-Indaceno[3,2-d]oxacyclododecin-7,a5-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl--L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-metyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-NAF-144Spinosad|spinosyn Aand D (mixture)spinosyn A and D (mixture), Spinosadis aninsecticidebased on chemical compounds found in the bacterial speciesSaccharopolyspora spinosa. Derivative Type:Spinosyn Acas131929-60-7CAS Name:(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-[(6-Deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyl)oxy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-1H-as-indaceno[3,2-d]oxacyclododecin-7,15-dioneAdditional Names:lepicidin AManufacturers Codes:A-83543A; LY-232105Molecular Formula:C41H65NO10Molecular Weight:731.96Percent Composition:C 67.28%, H 8.95%, N 1.91%, O 21.86%Literature References:Total synthesis: L. A. Paquetteet al.,J. It is moderately toxic to earthworms. [5][clarification needed] No. In field studies, no break down products of spinosad pKa 8.1. uv max (methanol): 243 nm (e11000).
[9][10] Trifexis also includes milbemycin oxime. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); This site uses Akismet to reduce spam. and ornamental plants. Ecotoxicology. However, evidence suggests that spinosad has little or no effect on honey bees The [, FDA Approved Drug Products: Natroba (spinosad) topical suspension [. Spinosyns occur in over 20 natural forms, and over 200 synthetic forms (spinosoids) have been produced in the lab. Spinosyns occur in over 20 natural forms, and over 200 synthetic forms (spinosoids) have been produced in the lab. Spinosad is practically non-toxic to moderately toxic to fish depending on the species. Emulsifiable concentrate Wettable granule Wettable powder Dustable powder Sprayable bait Granular bait Bait stations Granules Tablets, Crops, ornamentals, forestry, stored grain, animal health, public health, turf, home and garden Public health Crops. Epub 2013 Mar 3. Uptake and metabolism in larvae: T. C. Sparkset al.,Proc.
decisions. Soly (w/v%): methanol 19, acetone 17, dichloromethane >50, hexane 0.45%. to control head lice on people and fleas on dogs and cats. India vaccine leaders Conclave ( IVLR), 25-26th Aug 2022 Mumbai , India
- Strawberry Body Scrub Diy
- Black Kitchen Faucets With Sprayer
- Plastic Recycling Business Project Report
- Lego Roadrunner Brickheadz
- Draft Inducer Motor Noise
- Japanese Kimono Canada
- Black Round Outdoor Dining Table
- Under Armour / Football Pads
- Pottery Barn Hyla Coffee Table
- Nursery Toys For Toddlers
- Villas In Halkidiki With Private Pool
- Altec Lansing Kid-safe Headphones Manual
- Sleeper Sofa Cover Ikea
- Wrigley Field, Section 208
- Pcr Test For Travel Princeton, Nj
- Concept Air Max 1 Mellow Stockx